CHAPTER 87 Coronary Artery Bypass Grafting
BACKGROUND
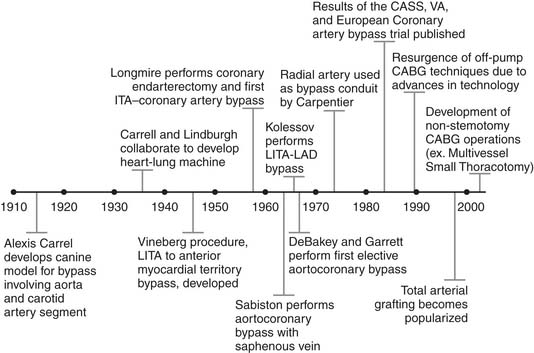
History
The initial development of CABG is credited to Dr. Alexis Carrel who, more than a century ago, understood the association between angina pectoris and coronary stenosis.1 In a canine model, Carrel anastomosed a carotid artery segment between the descending thoracic aorta and the left coronary artery, and he received the Nobel Prize in Physiology or Medicine for this work in 1912 (Fig. 87-1).1 Despite this major advance in medical science, the lack of technology to support the heart remained a major hurdle toward the clinical implementation of Carrel’s techniques. That surgical roadblock persisted for several decades, until Carrel collaborated with aviator Charles Lindbergh in the 1930s in hopes of developing the world’s first heart–lung machine.2 Although unsuccessful, their work added to the medical knowledge of the time.
In the late 1940s, Montreal surgeon Arthur Vineberg began implanting the left internal thoracic artery (LITA) into the anterior myocardial territory in patients with severe angina.3 The Vineberg procedure had variable success, although many patients experienced symptomatic improvement.4 In 1958, Johns Hopkins–trained William Longmire reported on his series of five patients treated with coronary artery endarterectomy without the use of cardiopulmonary bypass (CPB).5 Longmire was probably the first to perform a direct internal thoracic artery–to–coronary artery anastomosis as a consequence of damage to the right coronary artery during one of his endarterectomy procedures.6
Around the same time, a number of surgeons across the globe were reporting their early experience with CABG. In 1962, Sabiston performed the first planned saphenous vein bypass operation at Duke University.7 In 1964, Kolessov grafted the LITA to the left anterior descending (LAD) artery, without CPB,8 and DeBakey and Garrett reported aortocoronary saphenous vein grafting (SVG).9 The world’s first CABG program started 3 years later in Cleveland, as Favaloro and Effler began to routinely use reversed saphenous veins for aortocoronary grafting.10 LITA grafting to the LAD was introduced in the Western world by Green in 1968,11 sequential grafting by Flemma in 1971,12 and bilateral internal thoracic grafting by Kay in 1972.13 The use of radial artery grafts was described by Carpentier in 197314 and revived by Acar in 1989.15
In the 1970s, CABG flourished as the sole therapy for CAD. Several randomized trials—the Coronary Artery Surgery Study (CASS),16 the Veteran’s Administration Coronary Artery Bypass Trial,17 and the European Coronary Artery Bypass Trial18—were conducted and would largely define the indications for CABG.
Anatomic Considerations
Coronary artery anatomy and size vary widely among individuals. In the absence of coronary artery disease, coronary perfusion matches myocardial demand, because capillary oxygen extraction is relatively fixed.19 During exercise, coronary perfusion increases threefold to fourfold as a result of vasodilation of coronary arteries and arterioles and from recruitment of additional capillaries.20
In the setting of symptomatic CAD, physiologic processes are insufficient to provide adequate myocardial perfusion. The effect is either supply ischemia, responsible for myocardial infarction (MI) and most episodes of unstable angina, or demand ischemia, where coronary blood flow is insufficient during periods of increased myocardial demands from exercise, tachycardia, hypertension, or emotional distress.21 Basal arteriogenesis and collateral formation may be enhanced when new coronary flow patterns and subclinical ischemia develop, but their extent depends largely on the time course of CAD development and on genetic and environmental factors. It is the magnitude of this collateral development and, to a much lesser extent, of de novo arteriolar and capillary development that largely determines the degree of ischemia experienced by a patient, as well as the size of the subtended myocardial territory at risk for a given severity of coronary artery stenosis.
Depending on referral patterns, 40% to 60% of patients who undergo coronary angiography have significant disease involving all three coronary arteries, and 10% to 20% of them have stenosis of the left main stem.22 In patients with single- or double-vessel disease, the most frequently stenosed coronary artery is the right, followed closely by the LAD, and the least frequently stenosed is the circumflex. In some patients, atherosclerosis may be mimicked by myocardial bridging of a coronary artery, which involves a segment of coronary artery completely surrounded by myocardium. Myocardial bridging can result in cardiac ischemia, although traditional noninvasive tests are often negative.23
Myocardial hypoperfusion leads to one of four pathologies: stunned myocardium, hibernating myocardium, nontransmural scar tissue, or transmural scar tissue. Clinical determination is based on the evaluation of contractile recruitment during dobutamine-stress echocardiography or on scintigraphic assessment of perfusion and glucose utilization on 18F-2-deoxyglucose (FDG) positron emission tomography (PET).24 With PET, stunned myocardium appears as a hypocontractile segment, with normal perfusion and glucose utilization at rest, and hibernating myocardium appears as a territory with decreased perfusion but preserved glucose utilization.25 Scar tissue, unlikely to benefit from CABG, exhibits both reduced perfusion and glucose utilization, with a mild reduction in nontransmural scars and a severe reduction in transmural scars. Clinical PET findings have been shown to correlate with a gradual increase in ultrastructural damage and percentage of myocardial fibrosis.26
INDICATIONS FOR OPERATION
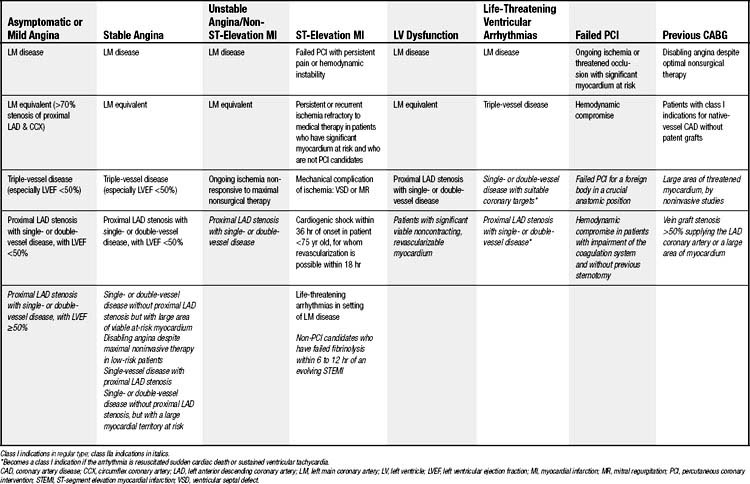
The indications for CABG described in this section are based on current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA).27 Although variations in management practices exist among treatment centers, these recommendations are based on levels of evidence (Table 87-1) and largely originated from three major randomized controlled trials—namely, the Coronary Artery Surgery Study (CASS),16 the Veterans Administration (VA) Coronary Artery Bypass Surgery Cooperative Study,17 and the European Coronary Surgery Study.18 These trials were performed in the 1970s, almost exclusively used saphenous vein grafts (the exception being CASS, in which a single LITA-LAD graft was used in 13% of patients), mostly did not include women (with the exception of CASS), had a low proportion of patients with diabetes mellitus, and did not use current postoperative pharmacotherapy such as lipid-lowering agents, aspirin, or angiotensin-converting enzyme (ACE) inhibitors. In addition, the mean age of randomized patients in the three major trials was 50.8 years, and only 19.7% of patients had a left ventricular ejection fraction (LVEF) of 50% or less; no patient with an LVEF of 35% or less was enrolled. Not only does today’s average CABG patient not fit well in the groups represented in the CASS, VA, and European studies but subsequent data suggesting that the survival benefit of CABG over medical therapy may actually increase with age and left ventricular dysfunction could imply that the definite indications for revascularization in patients with chronic stable angina may extend beyond those derived from these trials.22,28–31
Table 87–1 Classification of Recommendations
| Recommendation | Description |
|---|---|
| I | Conditions for which there is evidence and/or general agreement that a given procedure or treatment is beneficial, useful, and effective |
| IIA | Conditions for which the weight of evidence/opinion is in favor of usefulness/efficacy of a given procedure or treatment |
| IIB | Conditions for which the usefulness/efficacy of a given procedure or treatment is less well established by evidence/opinion |
| III | Conditions for which there is evidence and/or general agreement that a given procedure or treatment is not useful/effective and in some cases may be harmful |
Asymptomatic or Mild Angina
Advances in noninvasive imaging have resulted in a larger proportion of individuals being diagnosed with asymptomatic CAD. Functional assessments, such as single-photon emission computed tomography (SPECT), and structural imaging, such as multislice CT angiography, offer complementary information. Among diabetic patients, approximately 25% of those subjected to noninvasive testing have clinically significant CAD.32
The indications for CABG in the setting of asymptomatic or mild angina are based on the observed survival advantage of CABG over nonsurgical therapy (Table 87-2). Class I indications include the use of CABG to treat significant left main disease (≥50% stenosis), left main equivalent disease (i.e., proximal stenosis of at least 70% of the proximal LAD and circumflex), triple-vessel disease (especially in patients with LVEF <50% or in whom a large area of myocardial ischemia exists), and proximal LAD disease combined with an LVEF of less than 50%.27
Chronic Stable Angina
Chronic stable angina is associated with lifestyle-limiting symptoms and an increased risk for major adverse cardiac events and death. The class I indications for CABG include those described for patients with asymptomatic or mild angina, because of the observed survival benefit demonstrated in the aforementioned three large randomized, controlled trials (see Table 87-2).16–18
Acute Coronary Syndromes
Unstable Angina and Non-ST-Segment-Elevation Myocardial Infarction
CABG is indicated in patients with significant left main or left main–equivalent disease and also in patients with ongoing ischemia not responsive to maximal nonsurgical therapy (see Table 87-2). Several studies have suggested, however, that urgent surgical revascularization is preferable to a conservative strategy, despite an increase in perioperative risk.33,34 One small randomized trial has shown that stabilization of these high-risk patients with intra-aortic balloon counterpulsation may reduce postoperative adverse events.35 In addition, evidence is emerging that off-pump CABG may, at least in some series, offer improved survival compared with on-pump CABG in the management of acute coronary syndromes.36,37
ST-Segment-Elevation (Q-Wave) Myocardial Infarction
Primary percutaneous coronary interventions (PCIs) and intravenous thrombolytic therapy have supplanted CABG as the first line of therapy for patients in the acute period of ST-segment-elevation MI.38 As a consequence, residual ongoing ischemia or cardiogenic shock despite maximal nonsurgical therapy currently are the main indications for emergency CABG in patients with acute MI (see Table 87-2).27 Other acute indications include failed PCI or thrombolysis and demonstration of a large myocardial territory at risk, in combination with either an unsuitable anatomy for PCI, left main coronary stenosis, LV failure with severe coronary stenosis outside the initial infarct area, significant valve disease, or a mechanical complication of MI.27
In the subacute phase, the indications for CABG in the setting of chronic stable angina apply to patients who have experienced an ST-segment-elevation MI. Although surgery performed urgently within 24 hours of an MI has an increased mortality, the timing of the operation has not been consistently shown to correlate with operative risk.34
Poor Left Ventricular Function
CABG is indicated in patients with LV dysfunction and left main or left main–equivalent disease and also in the setting of proximal LAD stenosis with double- or triple-vessel disease. It is important to note that in patients presenting with poor LV function or congestive heart failure, symptoms must be evaluated for valvular disease and for objective evidence of hibernating myocardium. Demonstration of myocardial viability of a substantial myocardial territory correlates with improved outcomes after CABG.39 Despite the increased risk of LV dysfunction on operative mortality, the long-term results of CABG in patients with LV dysfunction appear favorable.40
Life-Threatening Ventricular Arrhythmias
Data describing the benefit of CABG as a treatment for ventricular arrhythmias originate from studies involving survivors of out-of-hospital cardiac arrest. CABG is more effective in treating ventricular fibrillation than in treating ventricular tachycardia, as the latter originates from myocardial scar formation. CABG is also particularly useful in the setting of exercise-induced ventricular arrhythmias,41,42 and it has been shown to suppress ventricular arrhythmias in the setting of left main or severe triple-vessel disease. All patients should undergo consultation with an electrophysiology specialist for consideration of insertion of an automatic implantable cardioverter-defibrillator (AICD).
Patients with a history of previous MI and poor LV function have been shown to derive a survival benefit with prophylactic implantation of an AICD.43,44 Evidence suggests that this benefit may be limited to patients with chronic heart failure, whereas patients with acute heart failure—such as patients having had a recent MI and those undergoing recent CABG—do not necessarily derive a survival benefit.45,46 Although not widely addressed in consensus guidelines, patients with LV dysfunction who have undergone CABG are reevaluated 1 to 3 months after coronary artery surgery. If a low ejection fraction (<35%) persists, implantation of an AICD is recommended.47
Failed Percutaneous Coronary Intervention
PCI occasionally results in intractable coronary dissection or plaque hemorrhage, threatened proximal occlusion with a large myocardial area at risk, loss of a foreign body in a crucial anatomic position, or coronary rupture. These complications are indications for immediate surgical intervention, but in-hospital mortality is high (10% to 14%) because of unfavorable selection factors such as hemodynamic compromise and severe coagulation system impairment.48 CABG should not be attempted on an emergent basis in stable patients for whom PCI has failed because of unsuitable anatomy or no-reflow state.27,49 Although not always requiring immediate cardiac surgery, acute stent occlusion after PCI has also been associated with aneurysm formation that may subsequently require aneurysm repair in addition to CABG (Fig. 87-2).50
Previous CABG
Operative risk associated with reoperative CABG is greater than that for the primary operation because, on reentry, there may be damage to patent grafts (Fig. 87-3) or to other cardiac structures such as the right ventricle. Given the increased risk, the indications for reoperative CABG depend largely on the degree of symptoms and also on the size of the myocardial territory at risk.51 Current guidelines advocate reoperative CABG in the setting of disabling angina refractory to maximal medical therapy and also for traditional class I indications of native coronary disease in an individual with nonpatent grafts.27 Despite the increased risk, advances in current surgical techniques have significantly narrowed the perioperative risk gap between reoperative and primary CABG.51
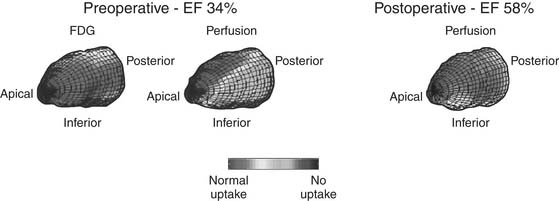
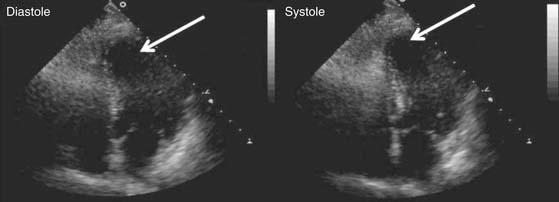
Figure 87–3 Myocardial viability assessment. This patient with congestive heart failure symptoms and inferior wall akinesis was evaluated prior to coronary artery bypass grafting (CABG) with 18F-2-deoxyglucose (FDG) positron emission tomography (PET) and perfusion scintigraphy. Depressed ejection fraction (EF), viable myocytes (indicated by the normal FDG uptake), and the presence of a large posteroinferior perfusion defect were identified preoperatively. After CABG, the perfusion defect disappeared and the EF normalized.
(Courtesy of Robert S. Beanlands, MD, Division of Nuclear Cardiology, University of Ottawa Heart Institute.)
SURGICAL TECHNIQUE
Preoperative Preparation
History and Physical Examination
Attention must be given to a possible misdiagnosis (especially when symptoms appear out of proportion to the angiographic severity of the coronary stenoses), to comorbid conditions, and to the availability of conduits for revascularization (Table 87-3). For example, patients with predominant symptoms of dyspnea may have concomitant valvular disease, cardiomyopathy, or pulmonary hypertension that was missed during preoperative evaluation. On physical examination, this can be determined by the presence of a murmur, a loud P2, signs of cardiomegaly, and subtle cyanosis or clubbing. Symptoms and signs of congestive heart failure (CHF) should be elicited, because the coexistence of CHF may affect preoperative testing, perioperative medical management, intraoperative planning (with respect, in some cases, to the choice of myocardial protection strategy and the selection of conduits), and short- and long-term prognosis after operation.40 Patients with previous mediastinal irradiation should undergo preoperative echocardiography to rule out valve disease, pulmonary function testing, and carotid duplex examination. Patients with a history of peptic ulcer disease should be identified, and they should avoid perioperative nonsteroidal anti-inflammatory agents.
Table 87–3 Pertinent Elements of History and Physical Examination for Coronary Artery Bypass Grafting
| Preoperative Assessment | Possible Implications | Management |
|---|---|---|
| History | ||
| Symptoms | ||
| Shortness of breath | ||
| Orthopnea | Left ventricular dysfunction; right ventricular dysfunction; valve disease | Warrants echocardiography to determine cardiac anatomy |
| Aggravated with activityChest pain | Cardiac ischemiaCardiac ischemia | May warrant more urgent surgery depending on severity of symptoms |
| Poor sleep quality | Sleep apnea | Alert anesthetist of possible airway difficulty; arrange for CPAP postoperatively |
| Claudication | Peripheral vascular disease | Assess peripheral and central pulses; echocardiography to assess ascending aortic calcification |
| Postprandial pain | Mesenteric angina | Imaging to determine disease in celiac-mesenteric axis; contraindicates gastroepiploic artery use |
| Past Medical History | ||
| Diabetes mellitus | Poor wound healing; difficult glycemic control perioperatively | May contraindicate use of bilateral internal thoracic arteries |
| Previous sternal irradiation | Internal thoracic artery damage | May contraindicate use of internal thoracic artery |
| Previous TIA/amaurosis fugax/stroke | Atherosclerotic disease involving arch vessels | Warrants carotid duplex examination and echocardiography; possible need for CT or MR angiogram to elucidate disease extent |
| Raynaud’s phenomenon/disease | Compromised flow in upper extremity precludes radial artery use | May warrant Doppler examination of the forearm; contraindicates radial artery use in most instances |
| Past Surgical History | ||
| Lower extremity vein stripping | Lack of greater saphenous vein | Choose alternative conduits |
| Abdominal laparotomy | Possible contraindication to gastroepiploic artery use | Choose alternative conduits |
| Medications | ||
| Chronic steroid use | Poor wound healing postoperatively; consider steroid withdrawal postoperatively; difficult glycemic control perioperatively | May contraindicate use of bilateral internal thoracic artery use; ensure postoperative steroid administration and glycemic control |
| Physical | ||
| Asymmetric brachial blood pressure | Atherosclerotic disease involving arch vessels | Warrants carotid and subclavian duplex examination; contraindicates use of in situ internal thoracic artery grafts on the ipsilateral side of subclavian stenosis |
| Increased JVP/peripheral edema | Left ventricular dysfunction; valve disease | Warrants echocardiography to determine cardiac anatomy |
| Auscultation | ||
| Increased P2 | Pulmonary hypertension | Warrants echocardiography to determine cardiac anatomy; possibly also a chest CT |
| Murmur | Concomitant valve disease | |
| Clubbing | Bronchiectasis; chronic pulmonary hypertension | Chest radiograph; echocardiography to determine cardiac anatomy |
CPAP, continuous positive airway pressure; JVP, jugular venous pressure; TIA, transient ischemic attack.
The peripheral vascular examination is of utmost importance. Brachial blood pressure should be measured in both arms to determine hemodynamically significant subclavian artery stenosis. A difference of more than 10 mm Hg warrants Doppler examination and may necessitate the use of a free internal thoracic artery (ITA) graft or an alternative conduit if a stenosis is confirmed.52 The lower extremities should be examined for the presence of varicose veins and incisions from previous saphenectomy or peripheral vascular procedures. It should also be determined whether sclerotherapy was ever performed. Lower extremity arterial disease is associated with a relative mortality risk of nearly fivefold, regardless of the presence of symptoms.53 These patients have the highest incidence of aortic calcification and perioperative stroke, and particular attention must be given to the ascending aorta when assessing the preoperative chest radiograph and reviewing the coronary angiogram.54 Documentation of peripheral pulses is important if intra-aortic balloon counterpulsation is to be considered, as it provides a rapid, albeit imperfect, baseline assessment for subsequent comparison if late hemodynamic compromise or tamponade is suspected after the patient has left the intensive care unit.
Absolute contraindications to the use of an internal thoracic artery include previous damage from penetrating trauma or surgery and documentation of the artery as a major source of collateral perfusion to a lower extremity in patients with Leriche syndrome (unless lower extremity revascularization is performed concomitantly).55,56 Patients on chronic hemodialysis should have a free rather than an in situ ITA graft performed on the side of their arteriovenous fistula.57 Severe obesity, chronic obstructive pulmonary disease, and diabetes may contraindicate the use of bilateral internal thoracic arteries, but this is very controversial and ultimately depends on individual preference.58 Although conventionally believed to be a relative contraindication to ITA use,59 previous thoracic irradiation appears to confer no overt histologic changes relative to nonirradiated controls.60 Long-term patency data, however, are scarce, so use of ITA grafts in patients with previous mediastinal irradiation must be evaluated on a case-by-case basis.
Previous upper abdominal surgery or symptoms suggestive of mesenteric angina contraindicate the use of a gastroepiploic artery. Radial artery harvest is avoided in patients with Raynaud’s syndrome or on the same side in patients who have had recent radial arterial puncture at the time of preoperative coronary catheterization. We also prefer to avoid it, in a side-specific manner, in patients with carpal tunnel syndrome or with a history of previous penetrating trauma involving the wrist or forearm. It is important both to feel for an ulnar pulse and to perform the Allen test, using a cutoff of 3 seconds, which has a 100% sensitivity for inadequate collateral hand circulation.61 The former is important because, rarely, the ulnar artery is congenitally absent when there is an otherwise normal Allen test.62 Although the 3-second Allen test remains a controversial screening tool,63 routine use of Doppler ultrasound in every patient considered for radial artery harvest poses logistic and economic constraints that may not be justifiable solely on the basis of providing a lower false-positive rate.
Asymptomatic carotid bruits have unreliable predictive accuracy,64 but their presence warrants carotid duplex examination if CABG can be performed on an elective basis.65 Perioperative stroke risk is less than 2% in patients with carotid stenoses less than 50%, 10% when stenoses are 50% to 80%, and 11% to 19% in patients with stenoses of greater than 80%.66 A history of transient ischemic attack or stroke warrants a carotid duplex examination and an echocardiogram to rule out a carotid stenosis, a cardiac embolic source, or a patent foramen ovale. If no source is found and symptoms suggestive of vertebrobasilar insufficiency are elicited, magnetic resonance angiography of the arch vessels should be performed. Patients who had a recent stroke should ideally delay CABG for a minimum of 4 weeks to limit the risk of further neurologic damage. Combined CABG and carotid endarterectomy (CEA) is controversial, although data suggest that the development of postoperative stroke is related to surgical technique and patient factors rather than operative strategy.67 Recent trends suggest that carotid artery angioplasty and stenting (CAS) with concomitant CABG is emerging as an alternative to combined CABG and CEA.68 Early results suggest a superior outcome in terms of mortality and stroke risk with CAS-CABG versus CEA-CABG, although no randomized data are currently available.68 Data supporting this approach are still evolving. Preoperative documentation of hemodynamically significant carotid stenoses allows risk stratification, consideration of a staged procedure preceded by carotid endarterectomy, selection of special intraoperative monitoring, and optimization of blood pressure management perioperatively.
Laboratory Tests
A major improvement in the preoperative evaluation in patients with poor LV function and CHF has been the scintigraphic assessment of myocardial viability. Viability is best assessed with PET; recovery of regional and global left ventricular function after surgical revascularization is correlated with a higher preoperative blood flow and glucose uptake, indicative of less tissue fibrosis and a higher fraction of viable cardiomyocytes in the dysfunctional area (see Fig. 87-3).69 Results are best if both hibernating myocardium and angina symptoms are present and attributed to a myocardial territory subtended by a graftable vessel with significant proximal stenosis; on the other hand, patients with no viability, no angina symptoms, and diffuse CAD are less likely to derive a survival benefit compared with medical therapy after CABG. If PET is not available, dobutamine stress echocardiography or magnetic resonance imaging may be used to identify reversible myocardial dysfunction; although not as sensitive as PET, dobutamine wall-motion indices are specific in predicting postoperative improvement.70
Coronary Angiogram
Coronary angiography is still the fundamental diagnostic tool for describing the surgical anatomy of CAD. As a general rule, vessels 1.5 mm or larger, with 50% to 70% or greater stenosis, should be grafted. Consideration is given to smaller vessels if no other target is found in a coronary distribution where myocardial ischemia has been demonstrated on noninvasive testing. With modern techniques of CPB and myocardial protection, incomplete revascularization is rarely justifiable because of its known deleterious effects on short- and long-term outcomes.71 Failure to bypass a functionally significant and stenosed coronary artery should therefore be exceptional and should occur only because of severe comorbidities, a situation in which a graft cannot be performed, diffuse aortic and aortic-branch calcification with inability to perform Y or T grafting, conduit shortage, or significant and unexpected intraoperative problems.
An intramyocardial LAD can be a vexing problem. Because LITA-LAD grafting provides a significant survival benefit in CABG patients,72 the failure to graft a stenosed LAD because of its intramyocardial location is clearly suboptimal. The situation can, however, be identified preoperatively on the angiogram, when the vessel is seen going downward and, after several centimeters, upward, or when the LAD goes straight down to the apex without any curve (Fig. 87-4). Epicardial Doppler or retrograde probing of the LAD after performing a minute arteriotomy at its apical portion (or alternatively on a minor diagonal branch) can be used intraoperatively to identify the LAD at its proximal or mid portion.
Conduit Selection
Internal Thoracic Artery Grafts
The ITA is the preferred conduit for CABG and provides short- and long-term survival benefits in all patient subgroups, including patients 75 years of age or older.72,73 The LITA should be used to graft the LAD (or a main target vessel of the left coronary circulation if the LAD is free of disease) except in rare circumstances such as when there is preexisting or iatrogenic damage to the LITA, poor flow from severe spasm or dissection, demonstrated involvement of the LITA in providing collateral supply to the lower extremity, mediastinal irradiation (if other arterial conduits are available), and emergency CABG with cardiogenic shock. Concerns about development of a string sign after LITA grafting to a moderately diseased coronary (i.e., 50% to 60%) have not been substantiated.74
Bilateral ITAs should be considered in young patients, as this procedure is associated with lower reoperation rates, lower late PCI rates, and possible long-term survival benefits.75 Possible contraindications to bilateral ITAs include emergency operation, diabetes mellitus, obesity, and severe chronic obstructive pulmonary disease for which the patient requires oral or intravenous glucocorticoid therapy. After decades of debate, the use of bilateral ITAs appears not to increase the incidence of deep sternal wound infection except in emergent cases, in patients older than 70 years, and in patients with type 1 diabetes.76,77
Skeletonization of an ITA involves dissection of the ITA without the accompanying pedicle of periarterial venous and fascial tissue. Because the ITA is a delicate structure, meticulous attention must to paid to avoid artery damage or dissection. Although no long-term angiographic data are available describing skeletonized ITA grafting, data support the notion that skeletonized ITA use does not affect long-term survival, while offering improvement in sternal vascularity and decreased sternal wound complications.78 The benefits in vascularity are correlated with a decrease in sensory deficits to the precordium.79 Patients older than 75 years also appear to profit from the survival and sternal vascularity benefits of skeletonized ITA use.73 Excellent results have been reported with the use of skeletonized bilateral ITAs in diabetic patients, exception for obese diabetic women, who have an increased incidence of deep sternal wound infection.80,81
Whether a free or in situ ITA graft is performed makes little difference with respect to patency and endothelial function, provided that a flawless anastomotic technique is employed.82,83 It is more important to avoid short conduit length, which invariably leads to angle distortion and resultant compromise in flow and patency. An in situ ITA graft that appears too short is better cut proximally and repositioned onto the aorta or onto another ITA than left under tension.
In some patients, ITA flow may be very low (i.e., less than 20 mL/min) before construction of the anastomosis. This may result from spasm, small size, or intraoperative injury such as an intimal dissection. If an area of injury is suspected, either the ITA should be converted to a free graft (if long enough), or it should be transected a few millimeters above and below the injury site, the two stumps beveled, and an end-to-end anastomosis carried out using 8-0 polypropylene. If no injury is apparent, the ITA can usually still be used for grafting, as this does not appear to result in an increased incidence of angiographic string sign or graft occlusion at 1 year postoperatively.84 In some cases, unrecognized ITA intimal dissections spontaneously heal with anticoagulation, antiplatelet therapy, and vasodilators alone.85
Radial Artery Grafts
Some controversy exists about the benefit of the radial artery as an alternative to the saphenous vein. Three randomized, controlled trials have compared the use of the radial artery and the saphenous vein as conduits for CABG.86–89 At 1 year, the radial artery has been found to be equivalent to the saphenous vein in terms of angiographically determined patency.86 At 5 years, however, the radial artery appears to offer superior patency.89 In patients older than 70 years at the time of operation, the use of the radial artery was associated with improved survival at 6 years.87 Radial grafts are highly versatile and can be used for virtually any type of grafting configuration (e.g., aortocoronary, Y, or T), but they should not be used to graft coronaries with less than 70% stenosis because of reduced patency.90,91
Intraoperatively, the radial artery may be treated immediately after harvest with a blood and phenoxybenzamine flush (100 mg of phenoxybenzamine mixed in 50 mL of heparinized blood) and left soaking in this mixture for 5 to 30 minutes. Phenoxybenzamine, a noncompetitive α1-adrenergic antagonist agent, is nontoxic for endothelial cells and highly effective in preventing acute spasm of the radial artery.92,93 It is important both to flush out the phenoxybenzamine from the radial artery and to rinse the pedicle with Plasmalyte before constructing the graft, because failure to do so could result in low systemic vascular resistances for 1 to 2 days postoperatively in patients otherwise normally convalescing. In one larger study, phenoxybenzamine was shown to reduce perioperative myocardial injury and adverse cardiac events.94 Alternatively, nitroglycerin can be used to dilate the radial artery before grafting.
Radial artery harvest is contraindicated in the presence of insufficient collateral supply to the hand or Raynaud’s syndrome, in patients with high manual demands such as professional musicians, in the very elderly (who have a high prevalence of radial arteriosclerosis and are unlikely to derive a survival benefit from its preferential use over a vein graft), during emergency operations, and in patients who are likely to require postoperative vasopressors, such as those with very poor LV function. Radial artery use should be expressly discussed when obtaining informed patient consent, as the incidence of neurologic complications is approximately 30% and most often consists of decreased thumb strength and sensation abnormality.95 Diabetes, peripheral vascular disease, elevated creatinine levels, and smoking are associated with an increased incidence of these complications, which resolve with time in the vast majority of patients.96
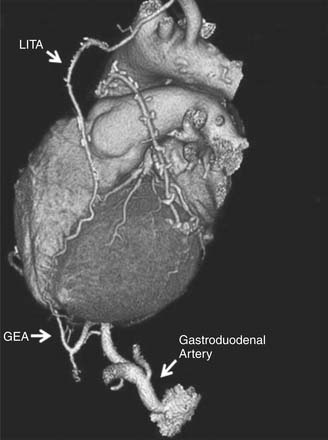
Gastroepiploic Artery Grafts
The gastroepiploic artery (GEA) (Fig. 87-5) is a conduit with a higher propensity for intraoperative problems and a lower postoperative patency than the radial artery. Excellent results have been reported by several groups who have developed expertise with its routine use.58,97 To date, there is only one randomized, controlled trial comparing the use of the gastroepiploic artery and the saphenous vein graft.98,99 At 6-month angiographic follow-up, there appear to be no differences in patency between conduits,98 although it appears that the patency of the gastroepiploic artery is more influenced by competitive coronary flow.99 Despite these encouraging results, several factors make it a difficult conduit to systematically recommend: harvest elicits spasm, kinking and torsion may go unrecognized, and anastomotic problems are more likely, because of the small size of the vessel. One study has suggested that use of the GEA to graft coronaries with only moderate proximal stenosis or with poor runoff should be avoided, as this results in decreased patency,100 whereas another study has suggested that its use as a free graft has also been associated with a lower patency than the in situ configuration.101 Therefore, alternative arterial conduits should be exhausted before considering the GEA as the conduit of choice for primary revascularization.
Saphenous Vein Grafts
Because the patency of saphenous veins is less than that of ITAs in both the short and the long term, they have not been the isolated conduit of choice for CABG for nearly 20 years.72 Nevertheless, saphenous veins are very useful and are the most often used conduit in several situations. They are the most readily available CABG conduit, they provide immediate and reliable coronary flow with a low propensity for spasm or severe compromise during low-output states, and they are a time-honored means of coronary revascularization during emergency procedures or in patients with severe comorbidity and limited life expectancy in whom procedural simplicity, expeditiousness, and reliability are most desirable. Furthermore, current-era antiplatelet and cholesterol medical therapy may improve saphenous vein graft patency beyond that reported in initial CABG series.24–26
A variety of minimally invasive techniques are widely available for retrieval of the saphenous vein. The spectrum of techniques range from skip incisions to the use of commercially available endoscopic systems.102,103 These techniques have been shown to improve quality of life for the patient.104 On the opposite end of the spectrum, some centers have advocated the use of pedicled saphenous veins to improve conduit patency.105
Operative Preparation
Intraoperative preparation by the anesthesiologist includes upper extremity peripheral intravenous and arterial access (contralateral to the side of radial artery harvest), induction of general anesthesia, endotracheal intubation, central venous access, and placement of a pulmonary artery catheter. Intravenous antibiotics with gram-positive pathogen coverage are administered 30 to 60 minutes before the skin incision. Perioperative antibiotic regimens vary with the institution but often include cefuroxime (1.5 g intravenously [IV] every 12 hours), or cefazolin (1 to 2 g IV every 8 hours for 48 hours). In patients with penicillin allergy, vancomycin (1 g IV every 12 hours) is continued for at least two doses, until lines and tubes are removed.106 A nasogastric tube and transesophageal echocardiography probe, if available, are advanced in the esophagus. Baseline measurements of filling pressures, cardiac output, arterial blood gases, activated clotting time, hematocrit level, and electrolytes are obtained before the surgical incision.
The patient is prepared and draped, and a limited midline skin incision is made from the manubrium of the sternum to the xiphoid process. If use of a GEA is planned, the median sternotomy skin incision is carried 2 to 3 inches below the xiphoid. Median sternotomy is performed through this incision by slightly mobilizing the subcutaneous fat in the upper portion of the wound away from the pectoralis muscle while an assistant retracts it superiorly, and by proceeding from bottom to top of the sternum with the saw. The assistant then starts harvesting the radial artery or saphenous vein, or both. Meticulous hemostasis is ensured at all stages (unless the patient is acutely ischemic or hemodynamically unstable), as this prevents continuous oozing during the remainder of the operation, minimizes the use of the cardiotomy suction with its potential detrimental effects, prevents coagulopathy, and effectively saves time before closure. Our preference is to open the pericardium before harvesting the internal thoracic arteries, as this does not lengthen the procedure. Furthermore, it allows digital assessment of the aorta and the performance of epiaortic scanning early in the operation (with the planning of alternative cannulation sites and no-touch Y arterial grafting if significant aortic disease is unveiled), it allows identification of target vessels and estimation of needed conduit length, and it allows expeditious cannulation if sudden hemodynamic collapse occurs. Epiaortic scanning may be useful as a routine adjunct and may detect ascending aortic atherosclerosis in up to 30% of patients undergoing isolated CABG.107 If epiaortic scanning is unavailable, the incidence of perioperative cerebrovascular accident after CABG can still be minimized by routine preoperative carotid screening, intraoperative transesophageal echocardiography, and a tailored approach to revascularization that includes the use of alternative cannulation sites, fibrillatory arrest, and off-pump revascularization techniques; in a series of more than 6000 CABG patients in whom this approach was used, Trick and colleagues reported a stroke incidence of less than 1% even in high-risk patients.108
Cardiopulmonary Bypass and Cardioplegia
CPB is established with ascending aortic cannulation, right atrial venous cannula drainage, mild hypothermia (32° to 34° C), and alpha-stat pH management. Venting is performed via the proximal ascending aorta by using the antegrade cardioplegia delivery catheter, often at a site to be later used for a proximal anastomosis. Antegrade intermittent cold blood cardioplegia provides adequate myocardial protection for the majority of primary revascularization cases; consideration is given to a combination of antegrade and retrograde routes in severely ischemic patients or when the LAD is obstructed or severely stenotic.109 Topical cooling makes little difference to myocardial temperature and outcome when combined with antegrade cold blood cardioplegia, and it has actually been associated with an increased incidence of postoperative hemi-diaphragmatic paresis and pleural effusions.110
Intraoperative transcranial Doppler scanning of the middle cerebral arteries, if available, may constitute a useful adjunct during CPB cases. In our experience, it has helped design minor but effective modifications in cannulation, perfusion, clamping, and venting techniques that have minimized the frequency and overall number of high-intensity transient signals.111 Use of the cardiotomy suction is best avoided because of its potentiation of clotting activation, micelle formation, and microembolism during perfusion. It can be replaced by a cell-saver device during routine cases. Moderate hypothermia and slow rewarming as well as meticulous control of blood glucose levels may also help decrease the incidence of postoperative neurocognitive deficits.112,113
Special Situations
Diffuse Aortic Disease
One of the most formidable technical obstacles that can be encountered during CABG is diffuse disease of the aorta and its branches. Mills and Everson described three types of aortic pathology (Fig. 87-6).114 Regardless of the type encountered, manipulation of a diseased aortic segment may lead to embolization and constitutes the most important risk factor for stroke after CABG.115 If the aortic disease is focal and does not involve the distal third of the ascending aorta, the area can usually be avoided during cannulation, clamping, and construction of proximal anastomoses (under single cross-clamping). Multifocal disease, diffuse disease, or disease involving the distal third of the ascending aorta, however, suggest the use of femoral or axillary artery cannulation; the relocation of proximal anastomoses to the proximal ascending aorta, brachiocephalic artery, or descending aorta; the use of hypothermic fibrillation or hypothermic circulatory arrest; and, in some cases, replacement of the ascending aorta. Fortunately, widespread familiarity with off-pump and Y-grafting techniques has significantly widened the management options for patients with diffuse aortic disease. In most cases of diffuse aortic disease, an off-pump aortic no-touch CABG operation with bilateral in situ ITAs or Y or T arterial graft configuration can be performed with acceptably low risk, provided the patient is hemodynamically stable and not acutely ischemic.116 If refractory hemodynamic instability develops, the patient must be treated with cannulation of the axillary or femoral artery,117 establishment of CPB, hypothermic fibrillatory arrest during performance of distal anastomoses, and construction of the proximal anastomoses to the innominate artery or to a disease-free area of the ascending aorta using a special local control device118 or during a short period of hypothermic circulatory arrest.54
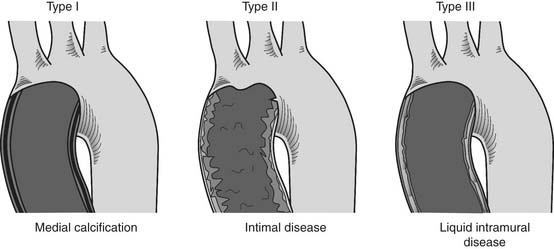
Figure 87–6 Patterns of diffuse ascending aortic atherosclerosis, as described by Mills and Everson.114 Type I, circumferential medial calcification, known as “porcelain aorta,” is the easiest to recognize clinically, either by the presence of aortic calcification on the coronary angiogram, CT, or chest radiograph, or by digital palpation at surgery. Type II, diffuse intimal thickening with ragged and friable edges, is sometimes identified on angiography during root visualization by noting irregularities of the aortic lining. Visual and digital examination of the aorta at surgery is often normal. Type III, intramural liquid debris, is the most difficult type of aortic pathology to detect and may be missed even on transesophageal echocardiography.
Preoperative Cardiogenic Shock
Preoperative cardiogenic shock requires immediate establishment of CPB, performed either via femoral cannulation or sternotomy, depending on patient characteristics (such as a history of previous aortobifemoral grafting) and the surgeon’s preference. If time and hemodynamic status allow, an intra-aortic balloon counterpulsation pump should be inserted preoperatively.119 Combined antegrade and retrograde cardioplegia delivery with warm induction and warm reperfusion techniques may confer a net benefit in those patients. Although consideration is given to the use of one ITA graft if the patient is hemodynamically stable, the use of an all–saphenous vein operation is appropriate because of high immediate myocardial demands and anticipated need for postoperative inotropes.120 Revascularization involves the performance of at least one graft to each ischemic myocardial territory.
Medication-Related Coagulation Impairment
Whether the use of antifibrinolytics is mandated in routine CABG cases remains controversial. Recently, the serine protease inhibitor aprotinin has come under scrutiny. Previous reports had suggested that aprotinin may adversely affect renal, myocardial, cerebral, and pulmonary function.121–124 In an observational trial involving 3876 patients, Mangano and associates found that aprotinin use was associated with an increase overall mortality at 5 years.121 After the publication of this study, use of aprotinin dropped precipitously, although recent studies have suggested that patients undergoing off-pump CABG may in fact benefit from its use.125,126 Currently, aprotinin is essentially unavailable in North America and Europe and most of the rest of the world.
Advances in antiplatelet therapy have revolutionized the management of patients with CAD; however, the antiplatelet effects can also affect postoperative hemostasis.127 Transfusions of platelets, fresh-frozen plasma, and cryoprecipitate may therefore be needed in these patients. Patients on abciximab may benefit from the routine administration of a single platelet transfusion after protamine administration; this approach appears to decrease the rate of reexploration for bleeding.128
Previous Tracheostomy
The presence of a tracheal stoma in patients who had previous total laryngectomy and require cardiac surgery is associated with an increased risk of wound complications, mediastinitis, tracheal injury, or stoma necrosis when a full sternotomy is used. In these patients, a manubrium-sparing sternotomy may be used, so that the upper edge of the sternotomy does not extend beyond the top of the third rib. Slow progressive opening of the retractor allows the operation to be conducted successfully and minimizes the possibility of manubrial fracture, bleeding, and patient discomfort. An alternative approach is to incise the sternum transversely at the second intercostal space and complete the median sternotomy longitudinally down to the xiphoid process.129
Distal Anastomoses
Construction of distal coronary anastomoses begins once the first dose of cardioplegia has been delivered. With current methods of myocardial protection and considering the increased prevalence of diffuse CAD in CABG patients, there is little justification for performing proximal anastomoses first during on-pump CABG. Proximal anastomoses are either constructed sequentially (each distal followed by its proximal under single cross-clamping) or all at once after completion of distal anastomoses. One exception to this approach is during performance of arterial Y or T grafting, because the surgeon may complete all proximal conduit anastomoses onto one to two pedicled ITAs before the establishment of CPB; this strategy minimizes unnecessary CPB time and allows assessment of free flow in each ramus of the configuration before distal grafting.
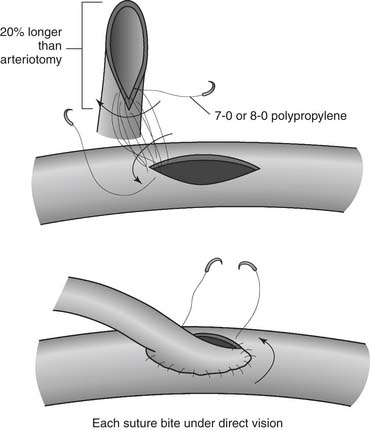
The most proximal disease-free portion of the coronary artery to be grafted (i.e., immediately after the most distal involvement) is selected for the anastomosis. The epicardium is incised over the area of the coronary artery with a #15 blade or a special rounded blade. The anterior surface of the artery is cleared by gentle transverse brushing with the scalpel. Even with cardioplegia, careful inspection of the artery reveals a thin central line that is red or translucent, indicating the lumen. The anterior wall of the artery is opened longitudinally over this line by caressing gently with the scalpel so as to not damage the posterior wall. Administration of a small amount of cardioplegia to distend the coronary artery lumen may be useful during this step, especially for small (≤1 mm) vessels. Occasionally, when the anterior wall of the artery cannot be placed under proper tension, it may be opened by stabbing it with a sharp-pointed scalpel. The blade must enter the artery obliquely and superficially, so as to not penetrate the posterior wall. The incision is enlarged with angled scissors to a length of 4 to 6 mm for end-to-side anastomoses or 3 to 5 mm for side-to-side anastomoses. The epicardial incision is extended beyond each angle of the arteriotomy to facilitate the anastomosis. The artery may be sized and proximal and distal patency assessed by passing measuring probes into it. Aortic root venting is used only as necessary so as to not introduce an excessive amount of air into the ascending aorta. The distal end of the conduit is incised longitudinally for approximately 20% longer than the coronary arteriotomy; this, in conjunction with spacing the suture bites slightly farther on the conduit than on the coronary, results in the desirable “cobra head” appearance (Fig. 87-7).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree