systems, with reversal of flow in the left coronary leading to a pulmonary-coronary steal. The left-to-right shunt is relatively small related to overall cardiac output but is significant considering coronary blood flow. Children with significant collateral circulation may survive past infancy; however, there is usually progressive left ventricular dysfunction. In a small percentage of children, the collateral vessels are enough to maintain adequate myocardial perfusion at rest and sometimes even during exertion allowing these patients to not present until adulthood.
Table 98.1 Origin of Both Coronary Arteries from One Sinus | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
chest X-ray, essentially due to the enlarged left atrium and left ventricle. It will look similar to dilated cardiomyopathy. In infants presenting with congestive heart failure, the electrocardiogram can be a useful diagnostic tool. Classically, these patients have developed lateral or anterolateral wall infarction with Q waves and ST segment elevation in leads I, aVL, and V4 to V6. While this electrocardiographic pattern can be found in other causes of myocardial infarction or cardiomyopathy, if this is seen in an infant in congestive heart failure, the diagnosis of ALCAPA needs to be strongly considered. As well, any infant presenting with dilated cardiomyopathy must be extensively evaluated to rule out ALCAPA. This diagnosis should also be considered in older children and adolescents with dilated cardiomyopathy because occasionally patients survive past infancy.
should be considered. Because of the high rate of early mortality and increased risk of late sudden death with this procedure, a variety of techniques were developed to create a dual coronary artery system, including coronary artery bypass grafting (CABG), aortopulmonary window, and direct reimplantation.
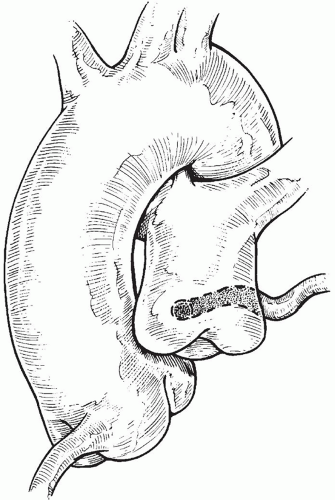
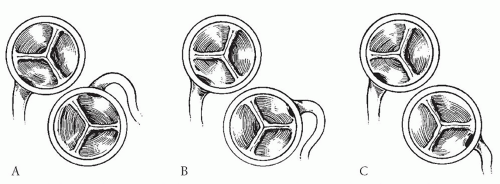
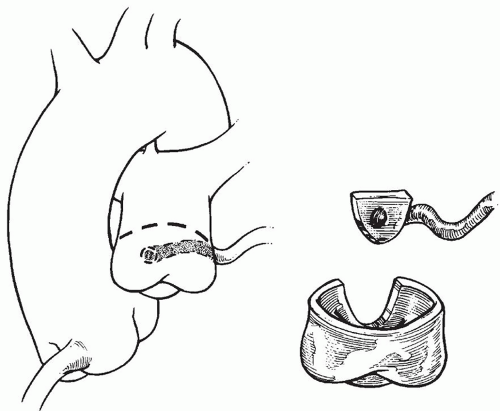
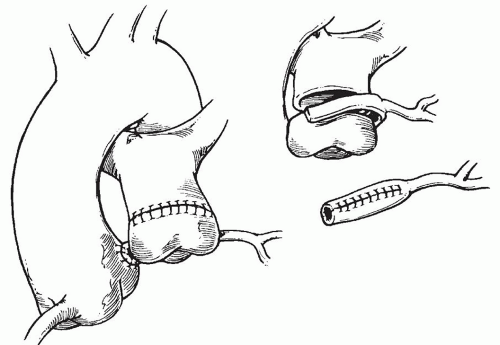
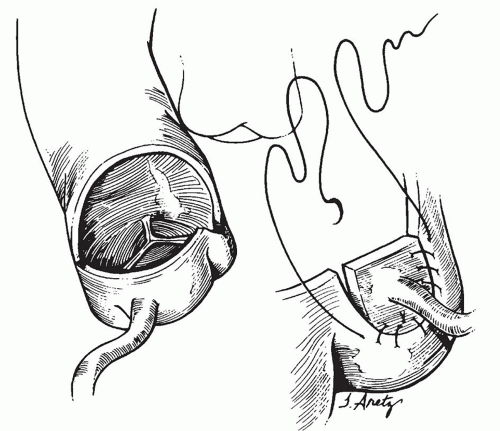
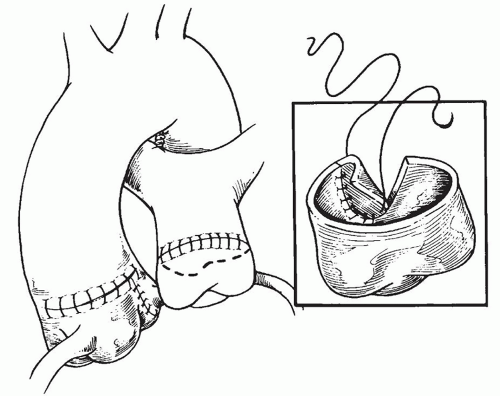
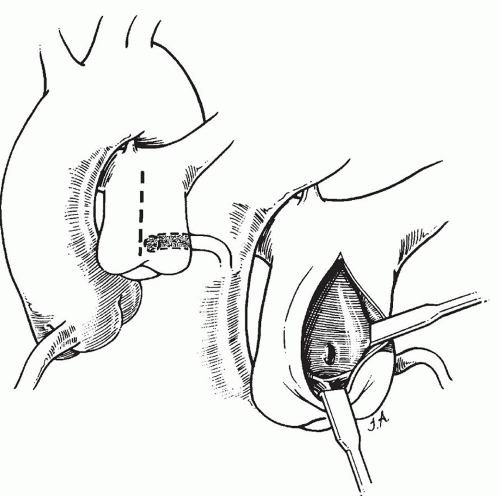
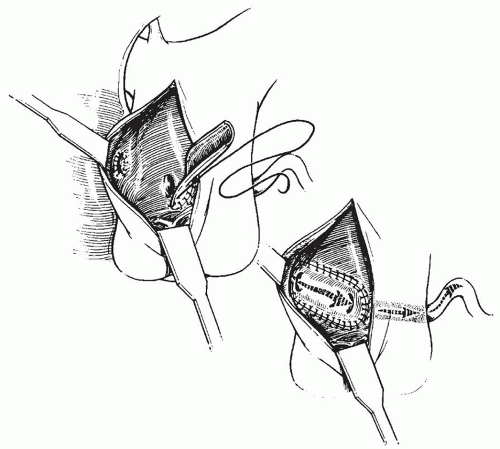
to occlude the branch pulmonary arteries and prevent run-off of cardioplegic solution into the lungs. Another way to prevent run-off is compression of the origin of the coronary artery from the pulmonary artery during administration of cardioplegic solution. A cannula is inserted in the ascending aorta for administration of cardioplegia solution. The aorta is then cross-clamped and cold cardioplegia is administered via the aortic root. If circulatory arrest is utilized, the head vessels are then occluded with tourniquets, the circulation is arrested, venous blood is drained into the reservoir, and the cannulae are removed. After adequate arrest, the pulmonary artery is transversely opened just above the sinotubular junction (Fig. 98.3). The anomalous coronary orifice is identified. The pulmonary artery is divided and the coronary ostium is excised from the pulmonary artery using a generous button of arterial wall, similar to the procedure used in the arterial switch operation. The segment of the pulmonary wall that is excised extends the proximal end of the coronary artery, which allows the aortic anastomosis to be accomplished without tension. The aortic commissure may need to be taken down to excise the coronary button if the coronary ostium is located near a commissure. If the coronary artery arises anteriorly from the pulmonary artery or from a branch pulmonary artery, the coronary artery can be extended using a tube constructed from pulmonary artery wall to allow reimplantation (Fig. 98.4). Using cautery, the proximal portion of the coronary artery is mobilized cautiously to avoid any small branches. Similar to the arterial switch operation, the aorta is then opened transversely just above the sinotubular junction and the incision is carried posteriorly above the left posterior sinus (Fig. 98.5). The sinus is then incised vertically to accept the coronary button. The coronary button is carefully aligned with the aortic incision to avoid twisting or kinking. Using a continuous suture of 7-0 polypropylene (Prolene), the anastomosis is started at the most inferior aspect of the coronary button, which is attached to the most inferior aspect of the incision in the sinus. The suture line is carried to the top of the incision anteriorly and posteriorly. The aorta is closed using a continuous suture of 7-0 Prolene, which is tied to the coronary button suture as the anastomosis is completed (Fig. 98.5). After the aorta has been closed, cardioplegia solution is administered and the anastomotic site is inspected for adequate filling of the coronary and hemostasis.
ischemia time, the cross-clamp may be removed prior to the pulmonary artery reconstruction. The left ventricle is inspected for adequate perfusion and function and the suture lines are inspected for hemostasis. Right and left atrial lines are placed to adequately monitor pressure and for drug administration. Atrial and ventricular pacing wires are also placed. After complete warming, the patient is separated from cardiopulmonary bypass. Careful attention to the electrocardiogram during reperfusion and after separation from bypass is necessary to evaluate for ischemia. Inotropic support may be temporarily needed due to preoperative left ventricular dysfunction.
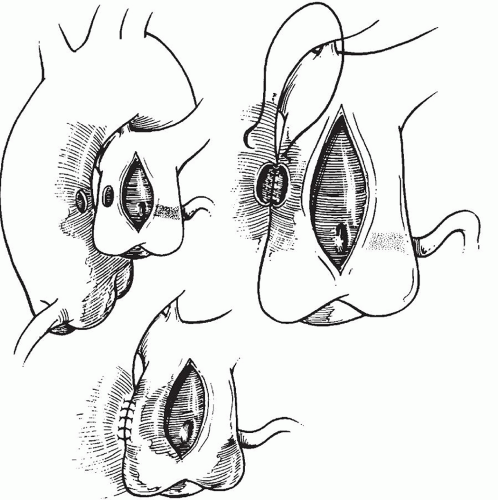
A4 mm PTFE tube graft is split longitudinally and customized to an appropriate length (Fig. 98.9). This graft acts as an intrapulmonary artery tunnel, baffling blood from the aortopulmonary window to the anomalous coronary ostium. The suture line starts at the anomalous coronary and is continued inferiorly along the pulmonary artery wall to the aortopulmonary window. The suture line is completed by returning to the coronary artery and finishing the superior aspect of the baffle. After the baffle is created, the pulmonary artery can be repaired using a prosthetic patch or autologous pericardium to avoid supravalvar right ventricular outflow tract obstruction (Fig. 98.10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree