There are limited data about the effectiveness of primary percutaneous coronary intervention (PPCI) for stent thrombosis treatment. We aimed to evaluate the prevalence and outcomes of PPCI in patients with ST elevation acute myocardial infarction (STEMI) due to stent thrombosis, and comparing the outcomes with patients treated for de novo coronary thrombosis. This was an observational cohort study of 2,935 patients who underwent PPCI from 2003 to 2011 with follow-up for a median of 3.0 years (interquartile range 1.2 to 4.6). The primary end point was the first major adverse cardiac event (MACE) defined as death, nonfatal myocardial infarction, stroke, or target vessel revascularization. Stent thrombosis overall accounted for 6.6% (194 of 2,935) of all STEMIs with a proportion that increased over time (3.3% in 2004 to 9.4% in 2011). A total of 34.5% were early, 30.9% late stent thrombosis, and 34.5% were very late stent thrombosis. Indications for the original intervention were elective in 27.8%, after acute coronary syndrome (non-STEMI or unstable angina) in 21.1%, and after PPCI in 51.1%. Patients with stent thrombosis had higher rates of hypertension, hypercholesterolemia, diabetes, renal dysfunction, and previous myocardial infarction or coronary artery bypass surgery compared with patients with native artery occlusion. MACE rates were higher in patients with stent thrombosis compared with patients with native artery occlusions (40.9%, 95% confidence interval [CI] 31.1 to 50.6 vs 15.1%, 95% CI 12.5 to 18.3; p <0.0001). The poor outcome of stent thrombosis was particularly associated with early and late stent thromboses. Very late stent thrombosis appears to be a relatively less serious event, with similar outcomes to native vessel thromboses (MACE very late stent thrombosis 16.5%, 95% CI 8.2 to 28.6 vs native 15.1%, 95% CI 12.5 to 18.3, p = 0.245). In conclusion, stent thrombosis accounts for an increasing proportion of STEMI and is associated with worse outcomes compared with native artery occlusion.
Stent thrombosis after percutaneous coronary intervention (PCI) occurs in 0.5% to 2.2% of cases, depending on the original indication for PCI, coronary anatomy and patient co-morbidities. Consequences of stent thrombosis may be devastating, with ST elevation myocardial infarction (STEMI) and sudden death as the most common presentations. Patients with stent thrombosis presenting with STEMI often undergo primary percutaneous coronary intervention (PPCI), but contemporary clinical data in this setting are limited. Despite the introduction of newer generation drug-eluting stents (DESs), which have decreased stent thrombosis rates, recent works have indicated that stent thrombosis is becoming an increasingly common cause of STEMI and is associated with adverse outcomes compared with de novo occlusions. We have undertaken a study of our PPCI cohort and have analyzed the incidence, clinical characteristics, and medium term outcomes of patients with STEMI due to stent thrombosis compared with patients with de novo coronary thrombosis.
Methods
This was an observational cohort study of 3,190 consecutive patients undergoing PPCI in a high volume center from January 2004 to July 2011. The London Chest Hospital is the only tertiary heart attack center for the northeast region of London and takes all patients with STEMI for PPCI in an unselected manner. This includes patients with cardiogenic shock and after cardiac arrest, including intubated and ventilated patients. The hospital serves a well-developed network of 6 local district general hospitals covering a population of 1.6 million and includes close working with the local London ambulance services. All patients undergoing PPCI are offered outpatient follow-up at our center. Patients with no follow-up data (150) were excluded from the analysis.
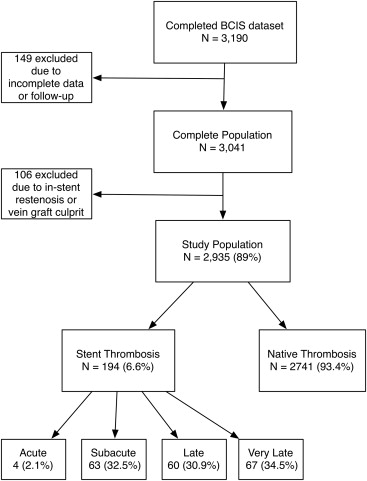
A total of 3,041 patients (95%) had complete electronic records and constituted the study population. One hundred six patients with either a vein graft culprit or restenosis were excluded resulting in a population of 2,935 patients ( Figure 1 ). Stent thromboses were defined according to the Academic Research Consortium definition and required angiographic confirmation of thrombus within, or 5 mm either side of, the stent. Further categorization was based on timing: early stent thrombosis (EST; acute [0 to 24 hours after stent placement] and subacute stent thrombosis [24 hours to 30 days]), late stent thrombosis (LST; 30 days to 1 year), and very late stent thrombosis (VLST; >1 year).
Standard PPCI protocol for our institution includes preloading with 300 mg aspirin, 300 mg (before 2007) or 600 mg clopidogrel (after 2007), and a bolus of glycoprotein IIb/IIIa receptor inhibitors, unless contraindicated. All patients were prescribed 75 mg aspirin and 75 mg clopidogrel maintenance therapy. Clopidogrel maintenance therapy was recommended for 1 year after PPCI. Aspiration thrombectomy was performed at the operator’s discretion. Successful PPCI result was defined as final Thrombolysis In Myocardial Infarction flow grade 3 and a residual stenosis of <20% in the infarct-related artery at the end of the procedure. During the study period, our center moved from using first-generation stents (predominantly CYPHER [Cordis Corporation, Miami Lakes, Florida] and TAXUS [Boston Scientific Corporation, Natick, Massachusetts]) to second-generation stents (Endeavour [Medtronic CardioVascular, Santa Rosa, California]). Data entered into the clinical database at the time of PPCI included patient characteristics and procedural complications. Postdischarge complications and further revascularization procedures were entered retrospectively from the electronic patient record and cardiac surgical database. Major adverse cardiac events (MACE) were defined as death, recurrent myocardial infarction (MI; defined as “new ischemic pain with new ST elevation or ischemic electrocardiographic changes and further elevation of enzymes (increase of creatine kinase-MB to ≥2× the reference value or increase in troponin T level of >30 ng/L [ninety-ninth percentile <10 ng/L]), whether treated with further revascularization therapy or not”), stroke, and target vessel revascularization. MACEs (identified from patient notes and electronic records) were adjudicated by 3 independent physicians who were not involved in the procedure and were unaware of the patient’s PPCI etiology (stent thrombosis or native occlusion). All-cause mortality was recorded to August 11, 2011 from the United Kingdom Office of National Statistics. A retrospective data quality audit of 100 randomly selected medical records established that 94.8% of data fields, including complications, were entered correctly into the database.
The data were collected as part of a mandatory national cardiac audit and all patient identifiable fields were removed before analysis. The local ethics committee advised us that formal ethical approval was not required.
Continuous variables are presented as mean ± SD and categorical variables as percentages. Categorical variables were compared among groups using the chi-square test or Fisher’s exact test when appropriate, whereas continuous variables were compared with the unpaired t test. Normality of distribution was assessed using the Shapiro-Wilk test. Cumulative incidences of MACE, death, and recurrent stent thrombosis after the index PPCI were estimated by the Kaplan-Meier method, and differences were assessed with the log-rank test. Cox regression analysis was used to estimate hazard ratios (HRs) for the effect of stent thrombosis in age-adjusted and fully adjusted models, based on significant covariates (p <0.05) associated with the outcome. A propensity score analysis was carried out using a nonparsimonious logistic regression model comparing patients with and without stent thrombosis. The full regression model was then used to generate the predicted probability of stent thrombosis for each patient (i.e., their “propensity score”), which ranges from 0 to 1. Model discrimination was assessed using the area under the receiver operating characteristic curve. We then undertook a regression adjustment incorporating the propensity score into a proportional hazard model as a covariate. We used SPSS for Mac, version 19.0 (SPSS Inc., Chicago, Illinois), for all analyses.
Results
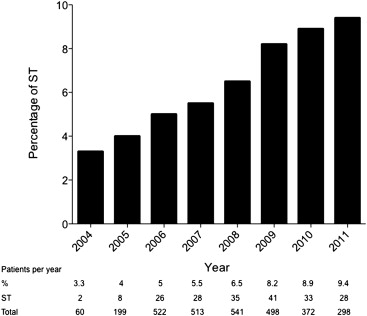
Stent thrombosis accounted for 6.6% (194 of 2,935) of all STEMIs with a frequency that increased over time (3.3% in 2004 to 9.4% in 2011; Figure 2 ). It was categorized as early in 34.5% (n = 67, acute in 2.1% [n = 4] and subacute in 32.5% [n = 63]), late in 30.9% (n = 60) and very late in 34.5% (n = 67).
The original implanted stent had been a DES in 43.3% (n = 84), bare-metal stent (BMS) in 55.2% (n = 107), and was unknown in 1.5% (n = 3) of patients. Of the 84 DES stent thromboses, 75 (89.3%) were first-generation DESs (sirolimus-eluting or paclitaxel-eluting stents) and 9 (10.7%) were second-generation stents (zotarolimus-eluting or everolimus-eluting stents). Stent thrombosis had occurred out to 6 years after BMS insertion and 4 years after DES insertion. EST (BMS 36.9% vs DES 32.9%) and LST (BMS 31.5% vs DES 28.2%) rates were similar between the stent types; there were higher although nonsignificant rates of VLST in the DES group (BMS 31.5% vs DES 37.6%). In the stent thrombosis group, the original stent deployment had been elective in 27.8% (n = 54) and urgent in the remainder (non-STEMI or unstable angina 21.1% [n = 41], STEMI 51.1% [n = 99]). At the time of stent thrombosis, 52.6% (n = 102) of patients were taking dual antiplatelet therapy, 44.3% (n = 86) were taking either aspirin or clopidogrel monotherapy, and 3.1% (n = 6) were taking no antiplatelet agents. Of those patients on 1 or no antiplatelet agents, 8 of 92 (8.7%) were because of premature discontinuation of 1 or both agents. Patients presenting with stent thrombosis had higher rates of baseline cardiac risk factors, previous MI, and previous coronary revascularization procedures (both PCI and coronary bypass surgery) compared with patients with native coronary thrombosis ( Table 1 ). Peak troponin levels were higher and left ventricular ejection fractions lower in patients with stent thrombosis than those with native coronary thrombosis ( Table 1 ). The clinical characteristics of the VLST group were different to the EST and LST groups. The VLST had lower rates of diabetes mellitus (VLST 22.5% vs EST 30.0% vs LST 27.1%, p = 0.003), renal failure (VLST 12.7% vs EST 28.6% vs LST 25.4%, p = 0.04), previous coronary artery bypass grafting (VLST 2.8% vs EST 10.2% vs LST 7.2%, p = 0.05), and shock (VLST 1.7% vs EST 5.6% vs LST 12.9%, p = 0.05) than those with either EST or LST. In fact, the VLST group had clinical characteristics that were similar to patients presenting with native coronary thrombosis (data not shown).
| Variable | Stent Thrombosis | Native Thrombosis | p |
|---|---|---|---|
| n = 194 (%) | n = 2,741 (%) | ||
| Men | 138 (71.0) | 2,091 (76.3) | 0.088 |
| Age (yrs) | 62.57 ± 17.1 | 63.09 ± 15.2 | 0.622 |
| Hypertension | 112 (57.5) | 1,031 (37.6) | 0.351 |
| Diabetes mellitus | 50 (26.0) | 458 (16.7) | 0.0001* |
| Hypercholesterolemia | 110 (55.0) | 811 (29.6) | 0.0001* |
| Previous MI | 120 (62.0) | 296 (10.8) | 0.0001* |
| Previous coronary artery bypass grafting | 6 (3.1) | 20 (0.7) | 0.001* |
| Cardiogenic shock | 12 (6.2) | 170 (6.0) | 0.682 |
| Left ventricular ejection fraction | 43.56 ± 7.2 | 45.11 ± 1.6 | 0.010* |
| Estimated glomerular filtration rate | 64.68 ± 15.5 | 74.13 ± 4.9 | 0.0001* |
| Peak troponin | 4.287 ± 9.0 | 2.823 ± 6.5 | 0.0004* |
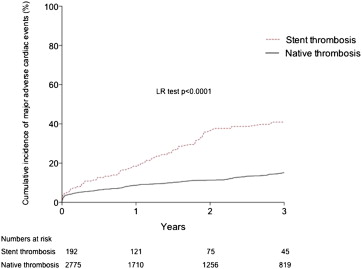
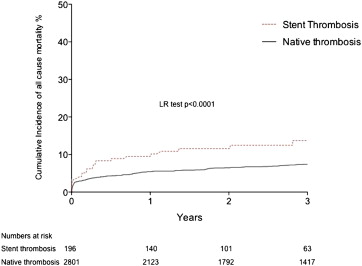
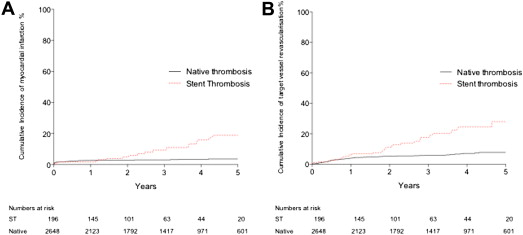
Procedural characteristics for patients with and without ST are listed in Table 2 . Additional stenting (168 [86.5%]), balloon angioplasty only (23 [11.9%]), or aspiration thrombectomy alone (3 [1.5%]) were used as emergency PPCI in patients with stent thrombosis. Rates of aspiration thrombectomy (47% overall) and glycoprotein IIb/IIIa inhibitor use (89% overall) were not different between the stent and native thrombosis groups. In-hospital MACE rates were significantly higher in patients with stent thrombosis compared with those with native thrombosis (16.9% vs 7.4%, p <0.0001; Table 3 ). Clinical follow-up was complete or out to at least 2 years in 82% of patients, with a median follow-up time of 2.9 years. MACE rates during the follow-up were also higher in patients with stent thrombosis (40.9%, 95% confidence interval [CI] 31.1 to 50.6 vs 15.1%, 95% CI 12.5 to 18.3, p <0.0001; Figure 3 ). This consisted of higher rates of all-cause mortality ( Figure 4 ), recurrent MI, and target vessel revascularization ( Figure 5 ).
| Variable | Stent Thrombosis | Native Thrombosis | p |
|---|---|---|---|
| n = 194 (%) | n = 2,741 (%) | ||
| Access route | |||
| Radial | 58 (29.9) | 1,184 (43.2) | 0.001* |
| Femoral | 136 (70.1) | 1,557 (56.8) | 0.001* |
| Anterior location of STEMI | 113 (58.2) | 1,253 (45.7) | 0.001* |
| Target coronary artery | 0.027* | ||
| Right | 65 (33.5) | 1,161 (42.4) | |
| Left main | 2 (1.0) | 6 (0.2) | |
| Left anterior descending | 109 (56.2) | 1,322 (48.2) | |
| Left circumflex | 18 (9.5) | 290 (10.6) | |
| Single-vessel disease | 94 (48.5) | 1,532 (55.9) | 0.002* |
| Multivessel disease | 100 (51.5) | 1,209 (44.1) | 0.002* |
| Door-to-balloon time (minutes) | 48.9 ± 28.8 | 52.3 ± 35.8 | 0.120 |
| Symptom-to-balloon time (minutes) | 252 ± 11 | 283 ± 15 | 0.230 |
| Multivessel intervention | 34 (17.5) | 343 (12.5) | 0.080 |
| Glycoprotein IIb/IIIa inhibitor | 173 (89.0) | 2,440 (89.0) | 0.963 |
| Thrombectomy use | 100 (51.5) | 1,291 (47.1) | 0.289 |
| Stent use | 168 (86.5) | 2,505 (91.4) | 0.061 |
| Success | 183 (94.5) | 2,629 (95.9) | 0.622 |
| Variable | Stent Thrombosis | Native Thrombosis | p |
|---|---|---|---|
| n = 194 (%) | n = 2,741 (%) | ||
| In-hospital | |||
| Major adverse cardiac events | 33 (17.0) | 203 (7.4) | 0.0001* |
| Death | 15 (7.7) | 96 (3.5) | 0.008* |
| Reinfarction | 8 (4.1) | 38 (1.4) | 0.010* |
| Reintervention PCI | 7 (3.6) | 49 (1.8) | 0.050 |
| Stroke | 1 (0.5) | 10 (0.4) | 0.411 |
| Emergency coronary artery bypass grafting | 2 (1) | 10 (0.4) | 0.411 |
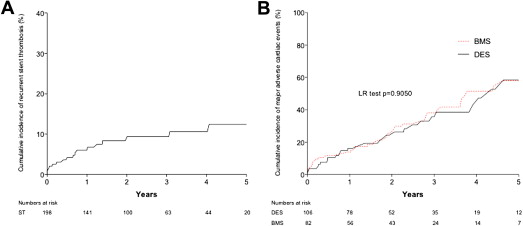
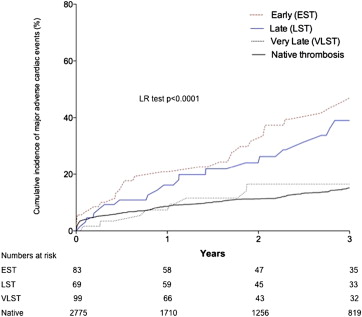
In the stent thrombosis group, cumulative incidence of recurrent stent thrombosis during follow-up after the index ST was 10.1% ( Figure 6 ). In the patients with stent thrombosis, there was no difference in long-term MACE between patients originally treated with either a DES or a BMS ( Figure 6 ). Cumulative incidence of MACE during follow-up was significantly lesser in patients with VLST (16.5%, 95% CI 9.8 to 24.6) compared with those with EST (46.0%, 95% CI 34.5 to 59.3, p = 0.01) or LST (39.0%, 95% CI 26.7 to 54.3, p = 0.04; Figure 7 ). There was no difference between patients with VLST and patients with native coronary thrombosis (16.5%, 95% CI 8.2 to 28.6 vs 15.1%, 95% CI 11.5 to 18.5, p = 0.245).
Stent thrombosis showed an association with MACE in age-adjusted Cox analysis ( Table 4 ; HR 2.59, 95% CI 1.98 to 3.37). After adjustment for confounding variables, the association of stent thrombosis with MACE was retained (HR 1.67, 95% CI 1.23 to 2.26), showing little change with further propensity score adjustment (HR 1.83, 95% CI 1.34 to 2.48). In addition to being an independent predictor of MACE as a whole, stent thrombosis was also an independent predictor of reinfarction (HR 2.61, 95% CI 1.51 to 4.71) and all-cause mortality (HR 1.51, 95% CI 1.04 to 2.46). Other independent predictors of MACE, reinfarction, and all-cause mortality are listed in Table 5 . Overall, stent thrombosis was an independent predictor of MACE, but differences exist based on the timing of the thrombosis. EST (HR 2.77, 95% CI 1.86 to 4.11, p <0.0001) and LST (HR 1.57, 95% CI 1.04 to 2.43; p = 0.041) were independent predictors of MACE after multivariate adjustment however VLST was not (HR 0.66, 95% CI 0.32 to 1.36, p = 0.263).



