Congenitally Corrected Transposition of the Great Arteries
Ergin Kocyildirim
Victor O. Morell
INTRODUCTION
Congenitally corrected transposition of the great arteries (ccTGA) is a complex cardiac lesion involving discordant atrioventricular (AV) and ventriculoarterial connections. The term congenitally corrected transposition was introduced by von Rokitansky in 1875. He described two patients in whom; the abnormal relationship of the great arteries was corrected functionally by the position of the ventricular septum. Cardell, Anderson et al., Lev and Rowlatt, and Schiebler et al. reported extensive anatomical descriptions and clinical studies. Anderson et al. described the anatomy of the conduction system. Alternative terms used to describe ccTGA such as L-TGA, for situs solitus (S), L-loop (L), and the aorta left to the pulmonary artery, and {I,D,D} for situs inversus with mirror image anatomy. D-loop is for D-transposed great arteries, where L means left and D means right. ccTGA have become a universal termination according to the Society of Thoracic Surgeons Congenital Heart Surgery Nomenclature and Database Project.
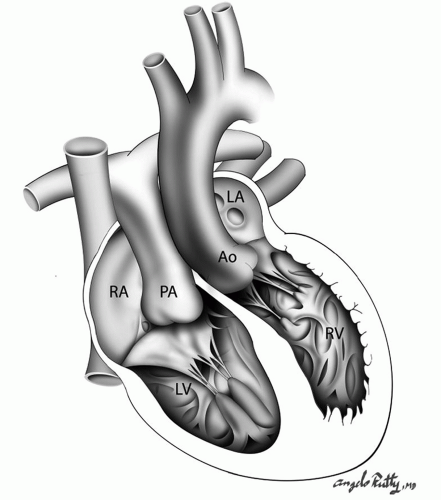
In this complex cardiac lesion, the right atrium is connected to the morphologic left ventricle, which is connected to the pulmonary artery and the morphologic left atrium is connected to a morphologic right ventricle, which is connected to the aorta (Fig. 90.1). As a result of the discordant connections at both levels, the blood flows in normal physiology. ccTGA represents the 1% of all congenital cardiac anomalies and about 94% of all cases are associated with other cardiac lesions, with the most common abnormalities involving the tricuspid valve (up to 91% of patients). Other associated defects are ventricular septal defects (VSDs), and pulmonary valve or subpulmonary stenosis resulting in left ventricular outflow tract obstruction (LVOTO). Also, dextrocardia is frequently associated with this cardiac defect. Most commonly, the associated lesions determine the severity of the symptoms and the surgical management strategy.
EMBRYOLOGY AND ANATOMY
During embryonic development, the primitive heart tube develops several curvatures. Corrected transposition in visceroatrial situs solitus develops when the primitive heart tube loops to the left instead of the right, resulting in the absence of spiral rotation of the conotruncal septum. This causes the aorta to connect to the morphologic right ventricle and the pulmonary artery to connect to the morphologic left ventricle. The normal process of septation and valve formation is also affected and the ventricular morphology is maintained consistent within the ventricular chamber. The AV valve and conduction tissue correspond to the overall morphology of each ventricle. The process of malseptation leads to a VSD, which is a frequent associated anomaly (50% to 80% of all cases). The VSD is usually perimembranous and large but can be located anywhere in the septum.
When atrial situs inversus is present, a mirror-image relation exists and the aorta is almost always to the right of the pulmonary artery with dextrocardia. Malalignment of the atrial and ventricular septa is present, except where the pulmonary, mitral, and tricuspid valves meet and are joined by the right fibrous trigone. The atrial septal attachment to the fibrous skeleton lies to the right of the ventricular septal attachment. These changes result in the abnormally positioned AV node and His bundle. Abnormalities of the AV node, including dual AV node with the abnormal His bundle, are quite common and many of those ccTGA patients develop complete heart block spontaneously during intra- or extra uterine life. Tricuspid valve and VSD surgery may also precipitate the complete heart block.
The left ventricular outflow tract in this cardiac defect is located in the region between the septal leaflet of the mitral valve and the muscular ventricular septum. Anatomic obstruction is significant and hemodynamically important in about 50% of the patients.
Tricuspid valve anomalies are noted frequently. When Ebstein anomaly occurs in the presence of ccTGA, the tricuspid valve and the right atrial morphology are different. Unlike single Ebstein anomaly, the anterior leaflet is not sail-like and the atrialized part of the right ventricle is small.
In atrial situs solitus, the right-sided coronary artery, which feeds left ventricle, usually gives rise to the anterior descending and circumflex branches. The left-sided coronary artery supplies the right ventricle and becomes the posterior descending artery. The most common variation of the coronary artery distribution is the existence of a single coronary artery that arises from the right-facing sinus and divides into right and left main branches. In ccTGA, there is also a tendency toward early branching of the left main.
 DIAGNOSIS
DIAGNOSISPatients with ccTGA may seek medical advice at any age, and their timing of presentation is usually dependent on the presence and severity of associated cardiac anomalies. Cyanosis could be the initial clinical presentation of a neonate or infant with severe pulmonary stenosis or pulmonary atresia requiring palliation with a systemic to pulmonary artery shunt. Not infrequently patients with pulmonary stenosis and a VSD have a nicely balanced circulation allowing for delayed surgical management during late infancy or early childhood.
Infants may experience congestive heart failure due to a large VSD, severe tricuspid regurgitation, aortic coarctation, or aortic arch abnormalities. Bradycardia can be the initial presenting symptom and it is most often caused by the spontaneous
development of complete heart block. The proportion of patients with heart block increases 2% per year to reach 30% by adult life. In the absence of associated cardiac anomalies, most often the defect manifests itself in older childhood or in the second decade, with the symptoms of exercise intolerance and cyanosis. In rare conditions, patients may go undiagnosed until late adulthood, when they present with symptoms of atypical chest pain and RV failure.
development of complete heart block. The proportion of patients with heart block increases 2% per year to reach 30% by adult life. In the absence of associated cardiac anomalies, most often the defect manifests itself in older childhood or in the second decade, with the symptoms of exercise intolerance and cyanosis. In rare conditions, patients may go undiagnosed until late adulthood, when they present with symptoms of atypical chest pain and RV failure.
The physical findings generally do not provide sufficient information to confirm the diagnosis of ccTGA. If tricuspid valve regurgitation is present, left parasternal lift and a systolic murmur akin to mitral regurgitation can be heard at the left sternal border. A leftward aorta and rightward posterior pulmonary artery can be present on the chest X-ray. The presence of AV block and a Q wave in the right precordial leads suggests the diagnosis.
The diagnosis of ccTGA is most often made by echocardiography. This study is useful to assess the ventricular morphology, mitral-pulmonary fibrous continuity, ventriculoarterial discordance, great vessels, and the associated cardiac lesions. Transesophageal echocardiography is also a useful tool in making diagnosis in adults.
Cardiac catheterization is useful in assessing ventricular morphology, systemic AV valve regurgitation, systemic ventricular function, and the presence and significance of any associated cardiac anomalies. Coronary angiography is indicated and important in older patients.
 SURGICAL TREATMENT
SURGICAL TREATMENTPalliative Operations
Patients in need of palliative surgery usually have either significant cyanosis or pulmonary overcirculation. In neonates and infants, significant pulmonary valve and/or subvalvar obstruction (LVOTO) in the presence of a VSD results in cyanosis. These patients can be initially managed with the placement of a modified Blalock-Taussig shunt.
Pulmonary artery banding could be utilized in neonates and infants with pulmonary overcirculation and failure to thrive secondary to the presence of a VSD. In these patients, the aim of the procedure is to reduce the distal pulmonary arterial pressure to <50% of the systemic pressure.
In patients who develop right ventricular failure after a classic repair pulmonary artery banding may be considered for LV retraining when a double-switch strategy is being considered. The ideal morphologic LV pressure after the banding should be approximately 75% of systemic. The development of morphologic LV dysfunction after the procedure is well recognized and could require postoperative inotropic support and/or loosening of the band.
Definitive Operations
The approaches to the surgical management of patients with ccTGA can be grouped into two options: physiologic (classic) or anatomic repairs. With a physiologic repair, the right ventricle remains the systemic ventricle and all other associated cardiac lesions are repaired. This approach is simpler but results in a RV-dependent systemic circulation, which has negative long-term implications, mainly RV failure and tricuspid regurgitation. After anatomic repair, the left ventricle becomes the systemic ventricle and the long-term outcomes are better. Nevertheless, the anatomic repair involves some form of a technically challenging double-switch procedure.
Closure of Ventricular Septal Defects
The majority of the VSDs in ccTGA are located in the perimembranous region. The presence of an overriding or straddling mitral valve usually signifies the presence of inlet extension. Most commonly, the surgical approach is via the right atrium with exposure of the VSD through the mitral valve. In selected cases, partial detachment of the septal leaflet of the mitral valve allows for better visualization of the defect. Transatrial approach is applicable to both physiologic and anatomic repairs. In patients requiring placement of a conduit between the morphologic LV and pulmonary arteries, the VSD could be easily closed through the ventriculotomy.
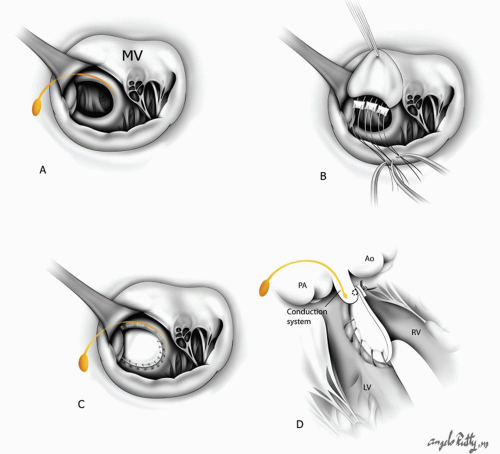
In the SLL type of L-TGA, it is important to realize that the conduction tissue runs in the subpulmonary infundibulum and along the anterior border of the defect. During VSD closure sutures must be placed on the left (systemic) side of the defect in order to avoid injury to the conduction system (Fig. 90.2). Frequently, the left-sided tricuspid valve has attachments to the posterior edge of the VSD and, therefore, the sutures need to be placed on the morphologic left ventricular side of the VSD.
The conduction system in patients with IDD type of L-TGA runs in the posterior edge of the defect. Therefore, VSD closure would require placement of all sutures along the pulmonary (left side) edge of the defect in order to avoid surgically induced complete heart block
Also, a VSD could be closed through the aortic valve if the aortic root is large. Sutures are placed on the left (systemic) side of the VSD. Extra care must be taken not to interfere with the chordae or the tricuspid valve leaflets, which may cause AV valve insufficiency. In patients with L-TGA, VSD closure is associated with a significant risk of complete heart block, either from suture damage to the conducting bundle or from retraction on the anteriorly placed AV node and bundle.
Repair of Left Ventricular Outflow Obstruction (Subpulmonary Obstruction)
Thirty to 50% of patients with L-TGA present with some form of pulmonary or subpulmonary obstruction. Direct relief of the LVOTO is usually not satisfactory because of the posterior position of the narrowed pulmonary outflow tract, which is wedged between the two AV valves. Accessory mitral valve chordal attachments to the subpulmonic area commonly contribute to the obstruction. Isolated valvular stenosis or discrete subvalvar membranes can be relieved by valvotomy and/or membrane resection. Another complicating factor is the presence of the abnormal conduction system in close proximity to the pulmonary valve in this patient population.
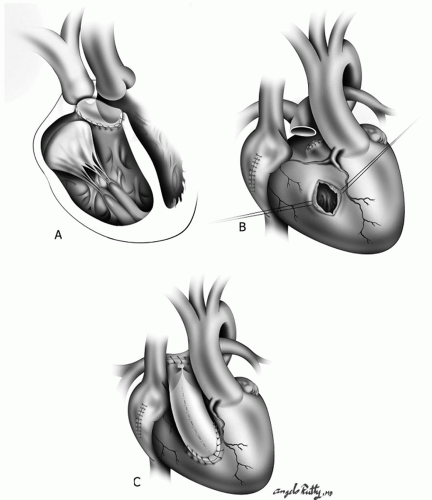
During a “physiologic repair,” the surgical management of LVOTO commonly involves the placement of an extra-anatomic conduit between the morphologic left ventricle and the pulmonary arteries (Fig. 90.3). The conduit takeoff from the LV is in a more apical position due to the location of the papillary muscles and coronary arteries. These conduits commonly sit retrosternally, an important fact to remember at the time of reoperation. Valved conduits are most
frequently used but a nonvalved synthetic tube can also be used when the pulmonary resistance is low and the distal pulmonary tree is adequate in size.
frequently used but a nonvalved synthetic tube can also be used when the pulmonary resistance is low and the distal pulmonary tree is adequate in size.
 Get Clinical Tree app for offline access 
|