CHAPTER 127 Congenital Anomalies of the Mitral Valve
EMBRYOLOGY OF THE MITRAL VALVE
The embryology of the mitral valve is complex. The understanding of the formation of the leaflets and suspension apparatus has evolved,1 and the current approach is based on immunohistochemistry, in vivo labeling of cushion tissue, and scanning electron micrograph of human and chick embryos.2 In humans, the mitral valve develops between the 5th and the 15th weeks of embryonic life. The leaflet and cordal tissue derive from the endocardial cushion tissue lying on the inner surface of the AV junction. The separation between atrial and ventricular myocardium is dependent on the sulcus tissue located on the epicardial side of the junction. As the cushion tissue elongates and grows toward the ventricular cavity, it becomes progressively delaminated from the underlying myocardium, and the leaflet transitions into a funnel-like structure completely attached to the myocardium. Then, perforations into the valve leaflet appear. The perforations grow and form the chordae tendineae. The atrial aspect of the cushion generates the spongy atrial layer, and the ventricular layer generates the fibrous part of the mitral valve and the cords. The development of the papillary muscle takes place at the same time and is originated from the myocardium. A horseshoe-shaped ridge lies in the left ventricle. Progressively, the anterior and posterior parts of the ridge lose contact with the ventricular wall. They will form the papillary muscles, and as they increase in size, they stay in contact with the cushion tissue at the tip of the papillary muscle. The midportion of the muscular ridge will be incorporated into the apical trabeculations of the left ventricle.3
PATHOLOGIC FEATURES
Supravalvular Mitral Ring
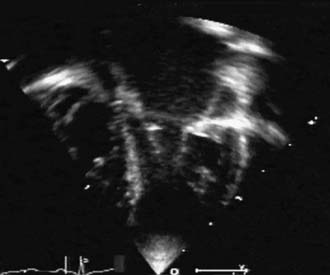
Often considered a congenital anomaly of the mitral valve, the supravalvular mitral ring is a fibrous construction attached to the posterior anulus of the mitral valve; it runs from both commissures to the mid-height of the anterior leaflet. The lesion is stenotic, often to a greater extent than might be suggested by the extension of the ring. This is more a result of the limitation of the opening of the anterior leaflet than of the actual diaphragm effect of the ring (Fig. 127-1). Strictly attached to the mitral valve anulus, it is to be differentiated from the cor triatriatum. Like the subaortic membrane in the left ventricular outflow tract, the supravalvular mitral ring is an acquired lesion resulting from turbulent flow through the mitral orifice. The primary lesion of the mitral valve responsible for the turbulent flow can be obvious, stenotic, and regurgitant, or it can be very discrete or mild and difficult to identify. It can be related to a prominent coronary sinus, as found in the left superior vena cava draining into the coronary sinus.4,5 It is perhaps for these reasons that the supravalvular mitral ring is prone to reoccur after surgical resection, unless the underlying anatomic anomaly has been identified and corrected.
Cleft Mitral Valve
The cleft mitral valve is very often isolated and can be easily differentiated from a left AV valve in a partial AV septal defect.6,7 It is an actual cleft with no suspension apparatus on the edges of the defect.8 The cleft is centered on the aortic commissure between the noncoronary and left coronary cusps.6 Each half of the anterior leaflet at the midportion bears the attachment of the strut cordae. Rarely, the cleft mitral valve is associated with a leaflet tissue defect, which is an acquired defect resulting from the regurgitation through the cleft. The defect is never stenotic and may generate only little regurgitation for a long time.
Lesions Associated with Lack of Valvular Tissue
Parachute Mitral Valve
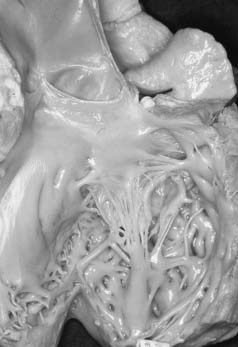
The parachute mitral valve can be found in isolation. It is, however, almost always associated with another cardiac anomaly, such as atrial septal defect (ASD), ventricular septal defect (VSD), or coarctation,9 and it is often integrated in Shone syndrome.10,11 It can also be seen in hypoplasia of the left ventricle, resulting in a univentricular palliation. The gross pathology shows a predominant single papillary muscle, with the orifice of the mitral valve overriding the tip of the papillary muscle. The spectrum of lesions for the suspension apparatus starts with complete fusion of the tip of the papillary muscle to the free edge of the valve.3 At the other end of the spectrum are cords that are relatively normal appearing, with good mobility of the leaflet (Fig. 127-2). The accessory papillary muscle is usually very small and devoted to a short segment of the free edge, or even to the undersurface of the leaflet tissue, as would be seen if it were a larger-than-normal secondary cord. The leaflet tissue can be intact or perforated.
The functional class depends on the interaction between the amount of tissue and the mobility of the leaflet; presence and size of the fenestrations; and presence, length, and quality of the cords.12 The parachute mitral valve almost always has a stenotic component, because the gradient is fixed but the valve grows. These patients may not require a valve operation.9
Papillary-Muscle-to-Commissure Fusion
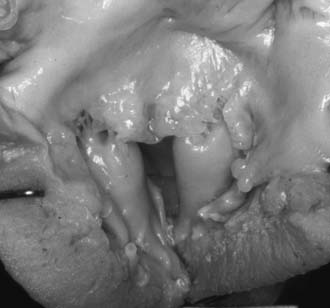
Fusion of papillary muscle to the commissure,8 also called short chordae syndrome, is defined by the presence of very short cords and a papillary muscle tip that is attached or fused to the commissural area of the free edge (Fig. 127-3). In the most extreme form, the cords are completely absent. The papillary muscles can be of normal size and volume. The valve is then generally more regurgitant than restrictive, which is because of the lack of valvular tissue and the restriction of the leaflet motion. When the papillary muscles are hypertrophied, the bulk of their mass is generally responsible for a predominantly restrictive valve.
Isolated Mitral Leaflet Hypoplasia
This very rare condition is almost restricted to the middle scallop of the posterior leaflet.8,13
Isolated Dilation of the Mitral Valve Anulus and Isolated Elongation of the Cords and Papillary Muscle
When the anatomy of the mitral valve is otherwise normal, it is difficult to ascertain the congenital origin of dilation of the mitral valve anulus and elongation of the suspension apparatus, but they are included in most studies of congenital anomalies of the mitral valve8,14 and account for 15% to 40% of the patients in published studies of congenital mitral valve regurgitation.12,14–16 However, there is no evidence of their congenital origin. Elongation of papillary muscles can be found at birth in the mitral or the tricuspid apparatus, but the muscles usually have an ischemic, beige aspect. Sometimes, the ischemic origin is demonstrated by acute rupture at or shortly after birth. Isolated dilation of the anulus is not found at birth.
Functional mitral regurgitations secondary to cardiomyopathies are not discussed here.
Accessory Mitral Valve Tissue
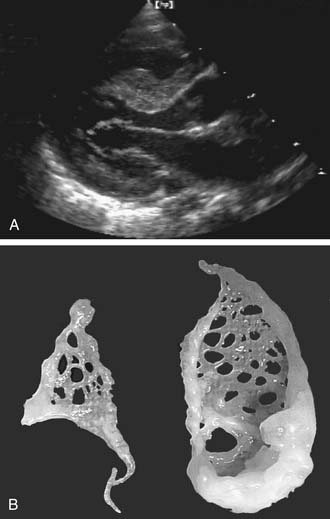
The intercordal spaces are filled with a dense network of valve tissue. When there is continuity between the anterior and the posterior leaflet, the accessory valvular tissue may generate a gradient directly related to the size of the perforations in the accessory tissue (Fig. 127-4).17 When the accessory valve tissue is entrapped in the left ventricular outflow tract, the mitral valve may become regurgitant because of the traction exerted by the accessory valvular tissue on the anterior leaflet, which results in the valve’s opening at mid systole18; however, in that case, the left ventricular outflow tract obstruction is the predominant hemodynamic lesion and is usually responsible for the diagnosis.19 Often, the accessory mitral valve tissue does not generate significant gradient or insufficiency.20
Mitral Valve Disease with Excess of Leaflet Tissue, and Mitral Valve Prolapse
It is debatable whether the mitral valve prolapse syndrome—in its most common form, limited to the middle scallop of the posterior defect—is congenital. In a large population of neonates,21 and using strict criteria, the incidence of mild bulging of the anterior leaflet was negligible, and no prolapses were detected. This tends to prove that mitral valve prolapse is an acquired disease. In its common form, it is the exception when encountered in neonates and infants. In adults, the histologic anomalies are limited to the middle scallop of the posterior leaflet, with predominant elastic fiber alteration and myxomatous tissue proliferation.22
The more extensive form of mitral valve prolapse is, however, seen in neonates and infants. In this form, an excess of tissue is distributed to both the anterior and posterior leaflets, and histologic examination reveals extensive infiltration of the spongiosa with myxomatous tissue. The histologic anomalies are identical to those found in patients with Marfan syndrome,23,24 Ehlers-Danlos syndrome, and osteogenesis imperfecta. Marfan syndrome is an autosomal dominant disorder with varying penetrance. The mutation is found on the fibrillin gene. Ehlers-Danlos syndrome is represented by a constellation of mutations linked to different subtypes.25 The extensive form of the mitral valve prolapse syndrome is encountered in sporadic cases, or in familial forms demonstrating autosomal dominant and X-linked inheritance. Three different loci, on chromosomes 16, 11, and 13, have been found to be linked to mitral valve prolapse, but no specific gene has been described.26
FUNCTIONAL CLASSIFICATION OF MITRAL VALVE ANOMALIES
Carpentier’s functional classification of mitral valve anomalies describes leaflet motion, regardless of the anatomy and the etiology.27 It is an essential tool for mitral valve repair and is best used together with echocardiography. It can also give significant clues to the lesions that will eventually be found during surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree