Conduction Disorders & Cardiac Pacing
Richard H. Hongo, MD
Nora F. Goldschlager, MD
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
Sinus node dysfunction (“sick sinus syndrome”)
![]() Sinus bradycardia: sinus rate of less than 60 bpm.
Sinus bradycardia: sinus rate of less than 60 bpm.
![]() Sinoatrial exit block, type I: progressively shorter P-P intervals, followed by failure of occurrence of a P wave.
Sinoatrial exit block, type I: progressively shorter P-P intervals, followed by failure of occurrence of a P wave.
![]() Sinoatrial exit block, type II: pauses in sinus rhythm that are multiples of basic sinus rate.
Sinoatrial exit block, type II: pauses in sinus rhythm that are multiples of basic sinus rate.
![]() Sinus arrest, sinus pauses: failure of occurrence of P waves at expected times.
Sinus arrest, sinus pauses: failure of occurrence of P waves at expected times.
Atrioventricular (AV) block
![]() First degree: prolonged PR interval more than 200 ms.
First degree: prolonged PR interval more than 200 ms.
![]() Second degree
Second degree
• Type I: progressive increase in PR interval, followed by failure of atrioventricular (AV) conduction and nonoccurrence of a QRS complex.
• Type II: abrupt failure of AV conduction not preceded by increasing PR intervals.
![]() “2:1 block:” due to lack of consecutive PR intervals; unable to assign either type I or type II block.
“2:1 block:” due to lack of consecutive PR intervals; unable to assign either type I or type II block.
![]() “Advanced:” AV conduction ratio ≥ 3:1.
“Advanced:” AV conduction ratio ≥ 3:1.
![]() Third degree (“complete”): independent atrial and ventricular rhythms, with failure of AV conduction despite temporal opportunity for it to occur.
Third degree (“complete”): independent atrial and ventricular rhythms, with failure of AV conduction despite temporal opportunity for it to occur.
 General Considerations
General Considerations
The clinical presentation of patients with conduction system disease is determined by two underlying abnormal conditions: the inability to maintain or increase the sinus rate in response to metabolic need, and atrioventricular (AV) dyssynchrony (inappropriately timed atrial and ventricular depolarization and contraction sequences).
 Pathophysiology & Etiology
Pathophysiology & Etiology
A. Sinus Node Dysfunction
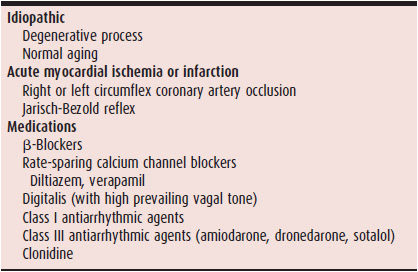
Sinus node dysfunction (“sick sinus syndrome”) is usually due to a degenerative process that involves the sinus node and sinoatrial (SA) area (Table 14–1). Often, the degenerative process and associated fibrosis also involve the approaches to the AV node and the AV node itself, as well as the intraventricular conduction system; as many as 25–30% of patients with sinus node dysfunction have evidence of AV and bundle branch conduction delay or block.
Table 14–1. Causes of Sinus Node Dysfunction
Respiratory sinus arrhythmia, in which the sinus rate increases with inspiration and decreases with expiration, is not an abnormal rhythm and is most commonly seen in young healthy persons. Nonrespiratory sinus arrhythmia, in which phasic changes in sinus rate are not due to respiration, may be accentuated by the use of vagal agents, such as digitalis and morphine, and is more likely to be observed in patients who are older and who have underlying cardiac disease, although the arrhythmia is not itself a marker for structural heart disease; its mechanism is unknown. Ventriculophasic sinus arrhythmia is an unusual rhythm that occurs during advanced second-degree or complete AV block; it is characterized by shorter P-P intervals that enclose QRS complexes and longer P-P intervals that do not enclose QRS complexes. The mechanism is not known with certainty, but may be related to the effects of the mechanical ventricular systole. The ventricular contraction increases the blood supply to the sinus node, thereby transiently increasing its firing rate; the resulting increase in intra-atrial pressure causes subsequent inhibition of the sinus rate. Ventriculophasic sinus arrhythmia is not a pathologic arrhythmia and should not be confused with premature atrial depolarizations or SA block. None of the sinus arrhythmias indicate sinus node dysfunction.
Sinus node dysfunction is present when marked sinus bradycardia, pauses in sinus rhythm (sinus arrest), SA block, or a combination of these exist (Figures 14–1 through Figure 14–5). Some clinically normal individuals who do not have structural heart disease can experience significant sinus bradycardia and prolonged sinus pauses under conditions of high vagal tone such as sleep. In some patients, a trigger, such as vomiting or coughing, can be identified; in other patients, high levels of acetylcholine may be responsible. Vagal stimulation, often from an identifiable trigger (Table 14–2), is commonly responsible for significant sinus bradyarrhythmias occurring in patients in an intensive care setting.
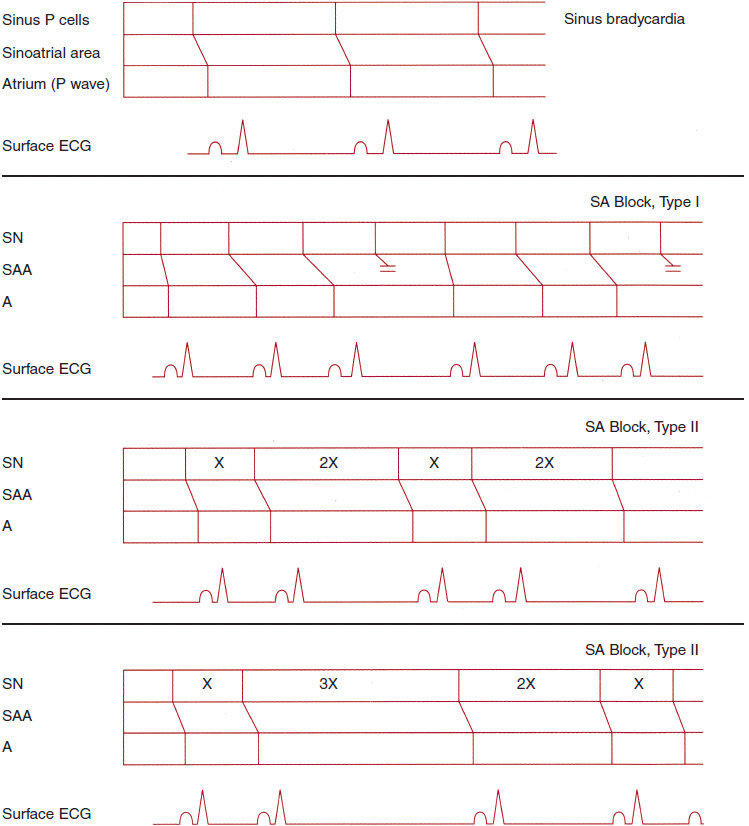
![]() Figure 14–1. Ladder diagrams illustrating sinus bradycardia and sinoatrial block, types I and II. ECG, electrocardiogram; SA, sinoatrial; SAA, sinoatrial area; SN, sinoatrial node.
Figure 14–1. Ladder diagrams illustrating sinus bradycardia and sinoatrial block, types I and II. ECG, electrocardiogram; SA, sinoatrial; SAA, sinoatrial area; SN, sinoatrial node.
![]() Figure 14–2. This 83-year-old woman was being treated for systolic heart failure and was receiving 200 mg/day of amiodarone for episodes of nonsustained ventricular tachycardia. She complained of profound effort fatigue but no symptoms of heart failure. Electrocardiogram reveals an atrial bradycardia at a rate of about 38 bpm. The P waves vary in morphology, suggesting some wandering of the atrial pacemaker. Left axis deviation and a left intraventricular conduction delay with ST- and T-wave abnormalities are present. The atrial bradycardia was presumed to be due to the amiodarone, which was discontinued, resulting in appreciable increase in a stable sinus rhythm, with amelioration of the patient’s effort fatigue.
Figure 14–2. This 83-year-old woman was being treated for systolic heart failure and was receiving 200 mg/day of amiodarone for episodes of nonsustained ventricular tachycardia. She complained of profound effort fatigue but no symptoms of heart failure. Electrocardiogram reveals an atrial bradycardia at a rate of about 38 bpm. The P waves vary in morphology, suggesting some wandering of the atrial pacemaker. Left axis deviation and a left intraventricular conduction delay with ST- and T-wave abnormalities are present. The atrial bradycardia was presumed to be due to the amiodarone, which was discontinued, resulting in appreciable increase in a stable sinus rhythm, with amelioration of the patient’s effort fatigue.
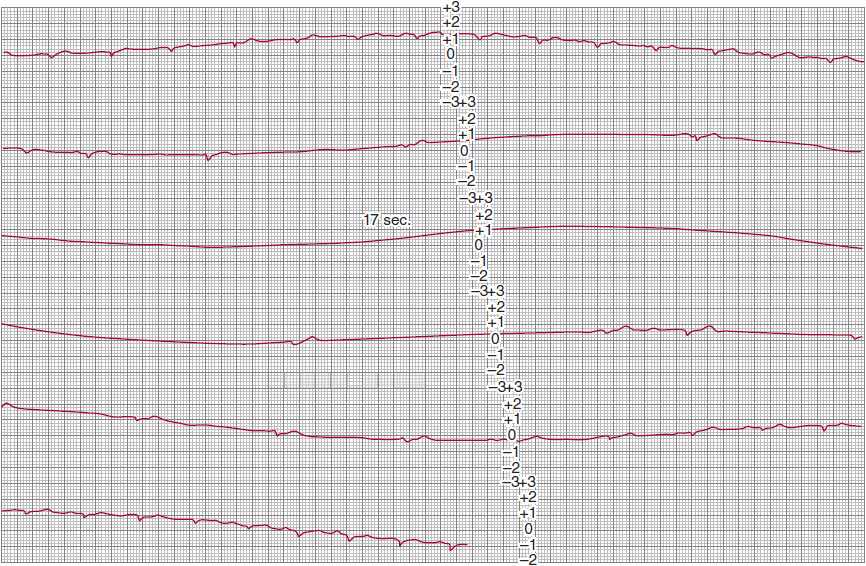
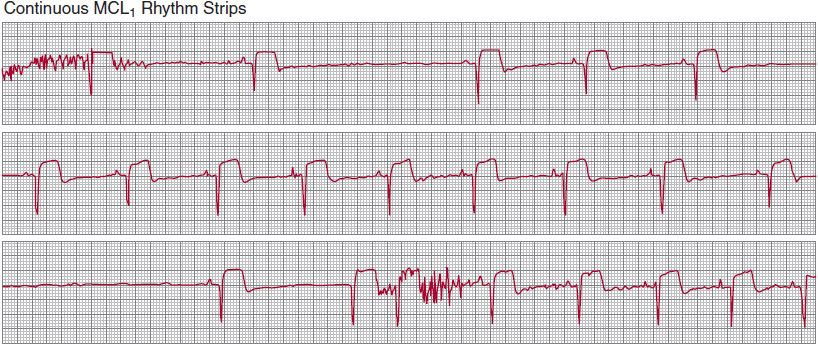
![]() Figure 14–3. Continuous modified lead-II ambulatory electrocardiographic recording in a patient with recurrent presyncopal spells. Sinus rhythm is present in the top strip; the second strip shows marked sinus slowing, followed by a 17-second period of sinus arrest without the appearance of a QRS escape rhythm. Sinus rhythm reappears in the fourth strip, gradually increasing its rate until stable rhythm is restored in the bottom strip. The absence of an escape rhythm raises the possibility of diffuse disease of the conduction system and impulse-generating tissue.
Figure 14–3. Continuous modified lead-II ambulatory electrocardiographic recording in a patient with recurrent presyncopal spells. Sinus rhythm is present in the top strip; the second strip shows marked sinus slowing, followed by a 17-second period of sinus arrest without the appearance of a QRS escape rhythm. Sinus rhythm reappears in the fourth strip, gradually increasing its rate until stable rhythm is restored in the bottom strip. The absence of an escape rhythm raises the possibility of diffuse disease of the conduction system and impulse-generating tissue.
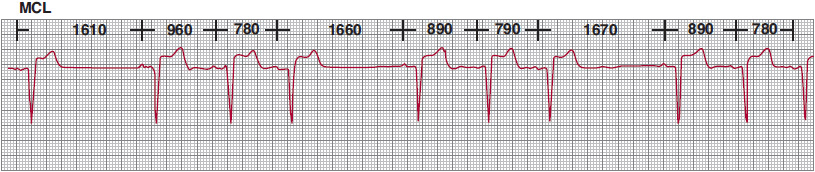
![]() Figure 14–4. Progressive decrease in P-wave cycle lengths followed by a pause in P-wave rate, indicating type I second-degree sinoatrial block. The pauses in sinus rate are less than twice the preceding sinus cycle lengths, satisfying the criteria for Wenckebach periodicity. MCL, modified chest lead.
Figure 14–4. Progressive decrease in P-wave cycle lengths followed by a pause in P-wave rate, indicating type I second-degree sinoatrial block. The pauses in sinus rate are less than twice the preceding sinus cycle lengths, satisfying the criteria for Wenckebach periodicity. MCL, modified chest lead.
![]() Figure 14–5. Irregular pauses in sinus rate, which occur abruptly and are not multiples of a basic sinus-cycle length. Best characterized as sinus pauses rather than sinoatrial block or sinus arrest, this rhythm indicates the existence of sinus node dysfunction. MCL1, modified chest lead.
Figure 14–5. Irregular pauses in sinus rate, which occur abruptly and are not multiples of a basic sinus-cycle length. Best characterized as sinus pauses rather than sinoatrial block or sinus arrest, this rhythm indicates the existence of sinus node dysfunction. MCL1, modified chest lead.
Table 14–2. Conditions Associated with Vagally Mediated Bradyarrhythmias
SA block may take the form of progressive delay in transmission of the sinus-generated impulse through the SA node to the atrium, finally resulting in a nonconducted sinus impulse and an absent P wave on the surface electrocardiogram (ECG) (Wenckebach, or type I second-degree exit block; see Figures 14–1 and 14–4), or abrupt failure of transmission of the sinus impulse to the atrium (type II second-degree exit block; see Figure 14–1). In type I second-degree exit block, there is less incremental delay with each successive impulse transmission through the SA nodal tissue (similar to type I AV nodal block); thus, the P-P intervals become progressively shorter until a P wave fails to occur. In type II second-degree exit block, abrupt failure of sinus impulse conduction to the atria can take the form of 2:1, 3:1 (and so on) SA block. Fixed 2:1 SA exit block cannot be distinguished from sinus bradycardia on the surface ECG.
Bradycardia-tachycardia syndrome is characterized by episodes of both bradycardias and supraventricular tachycardias (Figure 14–6). The bradycardia is due to sinus node dysfunction (sinus arrest or SA exit block) with associated junctional or ventricular escape rhythms. The supraventricular tachycardias may be atrial tachycardia, atrial flutter, atrial fibrillation, AV reciprocating tachycardia, or AV nodal reentry tachycardia (see Chapter 11); more than one type of tachycardia may occur in the same patient. Bradycardiatachycardia syndrome often represents diffuse conduction system disease, but is not necessarily associated with structural heart disease.
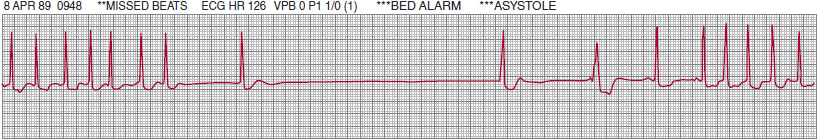
![]() Figure 14–6. Lead II rhythm strip characteristic of bradycardia-tachycardia syndrome, recorded from a patient with palpitations and intermittent dizzy spells.
Figure 14–6. Lead II rhythm strip characteristic of bradycardia-tachycardia syndrome, recorded from a patient with palpitations and intermittent dizzy spells.
Sinus bradycardia not uncommonly results from medications, particularly β-blockers, the rate-limiting calcium channel-blocking agents verapamil and diltiazem, and some commonly used antiarrhythmic agents such as sotalol, dronedarone, and amiodarone (see Figure 14–2). If these medications are necessary to treat the patient, permanent cardiac pacing is indicated.
The natural history of sinus node dysfunction is one of variable progression to an absence of identifiable sinus activity, with the process taking from 10 to 30 years. The condition itself is not associated with a high risk of arrhythmic death, although the morbidity caused by a sudden onset of bradycardia can be considerable. The ultimate prognosis for the patient with sinus node dysfunction typically depends on the presence and severity of underlying heart disease, rather than on the bradyarrhythmia itself.
B. Atrioventricular Block
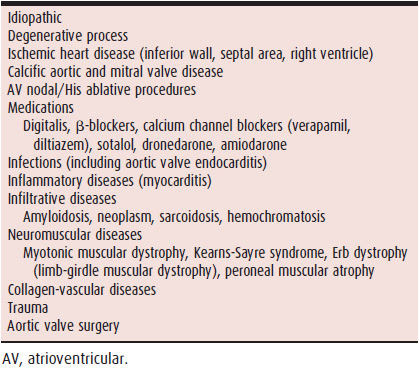
Delay or block can occur anywhere along the course of the AV conducting system that is made up of the AV node, His bundle, and Purkinje fibers (left and right bundle branches). Like sinus node dysfunction, AV nodal-His block and bundle branch block (BBB) are often the result of sclerode-generative processes. These processes can also involve the approaches to the AV node. Acquired AV nodal block is often due to acute ischemia and infarction (especially involving the inferior wall and right ventricle), infection, trauma, and medications (Table 14–3).
Table 14–3. Causes of Acquired AV Nodal-His Block
The AV node, or junction, is made up of three regions: atrionodal, central compact, and nodal-His. Cells of the atrionodal region have a relatively fast depolarization rate (45–60 bpm) and are responsive to autonomic nervous system input, whereas cells of the nodal-His region have a slower depolarization rate (about 40 bpm) and are generally unresponsive to autonomic influences. The site of origin of a junctional rhythm will therefore determine its rate, responsiveness to vagal and adrenergic input, and consequently the presence and severity of clinical symptoms.
The natural history of patients with AV block depends on whether or not there is an underlying cardiac condition with its own prognosis; however, the site of the block and the resulting rhythm disturbances themselves contribute to prognosis. First-degree AV block has little prognostic import. Persistent second-degree (types I and II), advanced second-degree (two or more consecutive nonconducted P waves), and complete AV block can all be associated with adverse outcomes, including death, unless the arrhythmias are vagally mediated or are due to other reversible causes.
 Clinical Findings
Clinical Findings
A. Symptoms & Signs
The symptoms resulting from conduction disorders reflect cerebral hypoperfusion, low cardiac output at rest or during exercise, and rarely, hemodynamic collapse. Symptoms, which are often subtle, can be episodic or chronic and can change over time. Because a patient often adapts activity levels to compensate for the impairment in heart rate response, significant symptoms may not be evident unless the patient is closely questioned about specific activities and effort tolerance, or the clinician actually observes the patient during performance of activities of daily living such as walking or during formal treadmill exercise tests.
Syncope is the classic symptom of cerebral hypoperfusion due to bradycardia; however, symptoms of presyncope such as dizziness, lightheadedness, and confusion can reflect the same pathophysiology and warrant the same aggressive approach to diagnosis and management. It should be emphasized that patients with cerebral hypoperfusion often have impairment of memory surrounding the presyncopal or syncopal episodes and may therefore be unable to provide an adequate or accurate history surrounding the events; witnesses, if present, can contribute significant information in these cases.
Patients with sinus node dysfunction or AV block, in whom the escape pacemaker is unresponsive to autonomic nervous system input, cannot increase their heart rate in response to increases in oxygen demand. They are, therefore, intolerant of effort and will report symptoms of exercise-related breathlessness, weakness, and fatigue. These symptoms, which can be disabling, are often confused with other conditions such as hypothyroidism, medications, underlying heart disease, deconditioning, or simply older age.
During periods of AV block, the atria and ventricles often depolarize and contract asynchronously or with inappropriate and suboptimal timing between atrial and ventricular contractions. There are variable increases in atrial pressures and volumes depending on the degree to which the AV valves are open or closed at the onset of ventricular systole. The resulting atrial stretch and secretion of atrial natriuretic peptide produce reflex systemic hypotension and cerebral hypoperfusion. In addition, the increases in left atrial and pulmonary venous pressures can cause shortness of breath and pulmonary venous congestion, including frank pulmonary edema. The mistaken diagnosis of refractory left ventricular dysfunction is not infrequently made in this situation.
Patients who have the bradycardia-tachycardia syndrome (see Figure 14–6) have symptoms referable to both the bradycardia and the tachycardia. During tachycardia, the patient can experience uncomfortable palpitations, breathlessness, chest discomfort, and at times, symptoms of cerebral hypoperfusion from excessively rapid heart rates.
More rarely, bradycardias can lead to a potentially lethal form of polymorphic ventricular tachycardia known as bradycardia- or pause-dependent ventricular tachycardia (Figure 14–7); this tachycardia is often triggered in the setting of QT interval prolongation brought on by the longer R-R intervals associated with either bradycardia or pauses (torsade de pointes ventricular tachycardia). Symptoms in these patients can include not only palpitations, presyncope, and syncope, but also cardiac arrest.
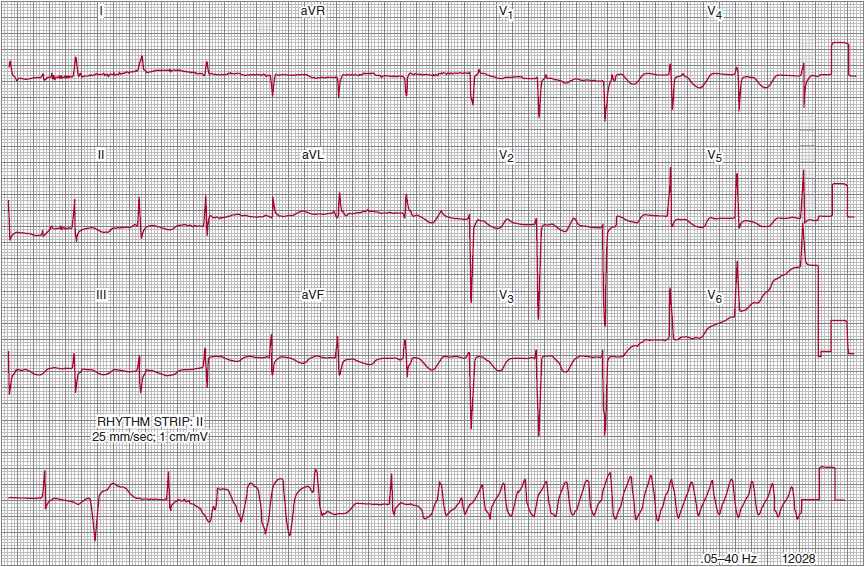
![]() Figure 14–7. Pause-dependent ventricular tachycardia causing loss of consciousness in a patient with syncopal and presyncopal spells. Note the markedly prolonged QT interval, which is longer and more bizarre when associated with the longer RR cycle lengths.
Figure 14–7. Pause-dependent ventricular tachycardia causing loss of consciousness in a patient with syncopal and presyncopal spells. Note the markedly prolonged QT interval, which is longer and more bizarre when associated with the longer RR cycle lengths.
B. Physical Examination
The physical examination of the patient with bradycardia reflects the origin of the QRS rhythm and the AV relationship more so than the heart rate per se. Junctional or ventricular escape rhythms resulting from atrial bradycardia or AV block produce AV dyssynchrony. This dyssynchrony results in varying degrees of atrial contribution to ventricular filling, as well as varying stroke outputs and systolic blood pressures. Because AV dyssynchrony causes changes in the positions of the mitral and tricuspid valves relative to their fully closed or open positions, the intensity of the first heart sound will vary, as will the audibility of atrial gallop (S4) sounds and the intensity of semilunar valve systolic ejection murmurs and AV valve regurgitant murmurs.
Examination of the venous pulse contour in the neck can reveal cannon a waves (due to atrial systole occurring against closed AV valves) and prominent cv waves (due to ventricular contraction occurring with the AV valves partially or even completely open), and the diagnosis of AV block can occasionally be made by recognizing these findings. Central venous pressure elevation (not to be confused with a and cv waves) is a common physical finding that is independent of the venous pulse contour.
The carotid pulse may vary in volume and upstroke velocity in patients with AV dyssynchrony. Examination of the chest may disclose rales, which reflect increased pulmonary venous pressure and valvular regurgitation rather than systolic or diastolic ventricular dysfunction. The liver may be enlarged and may pulsate because of transmitted a and cv waves. Peripheral edema may also be present if the AV dyssynchrony is chronic.
These same physical findings can also occur in patients who have sinus rhythm but develop AV dyssynchrony from being paced by a single-chamber ventricular system. In these patients, symptoms of weakness, fatigue, and congestive heart failure, together with physical findings indicating AV dyssynchrony, constitute the “pacemaker syndrome,” and this syndrome is treated by upgrading the single-chamber ventricular pacing system to a dual-chamber system in which sensing of the atrial rhythm triggers a paced ventricular response to restore AV synchrony (see later section, Permanent Pacing).
In addition to the findings described earlier, marked bradycardia (< 40 bpm) may result in ventricular dilatation in order to use the Frank-Starling effect to boost stroke volume and increase cardiac output despite the slow heart rate. This dilatation may lead to left ventricular gallop sounds and regurgitant murmurs, which disappear when normal heart rate is returned. Also, the enlarged ventricles may be palpable.
C. Diagnostic Studies
1. Sinus node dysfunction
A. ELECTROCARDIOGRAPHY—The P waves inscribed on the surface ECG represent atrial depolarizations. Sinus node depolarization precedes atrial depolarization and is not seen on the surface ECG. The P waves that result from sinus-generated impulses must be inferred from their morphology and axis.
The sinus P wave has a mean frontal plane axis of +15 to +75° and is upright in leads I, II, and aVF, inverted in lead aVR, and variable in leads III and aVL. In the horizontal plane, the sinus P wave can be inverted in lead V1 but is upright in leads V3–V6. Respiratory variation in the sinus P-wave contour can be seen in the inferior leads and should not be confused with wandering atrial pacemaker, which is unrelated to breathing. Sinus arrhythmia is present when the P-wave morphology is normal and consistent and the P-P intervals vary by more than 160 ms. SA block exists when some impulses generated by the sinus pacemaking cells do not exit the SA node to depolarize the atria; in the absence of atrial depolarization, a P wave will not be inscribed on the surface ECG. High-degree (advanced) SA block, in which most of the sinus impulses fail to exit the SA node to the atrium, is inscribed on the surface ECG as pauses in sinus rhythm. These pauses often cannot be differentiated from sinus arrest caused by failure of impulse generation by the sinus node. If the pauses between sinus P waves are multiples of a basic P wave rate, however, the diagnosis of type II second-degree SA block can be made.
B. ELECTROPHYSIOLOGIC STUDIES—Sinus node function can be evaluated in the electrophysiology laboratory by means of simultaneous surface and intracardiac electrographic recordings made during basal conditions, physiologic and pharmacologic interventions, and atrial pacing. This evaluation can be undertaken in patients with either symptomatic sinus bradycardia or bradycardia-tachycardia syndrome. It can also be used in patients with recurrent syncope of unclear etiology, although the diagnostic yield and predictive value for the condition is limited. Measurements include the intrinsic heart rate, the sinus node recovery time (SNRT), the SA conduction time (SACT), and the response to parasympathetic (vagal) stimulation as assessed by carotid sinus massage.
The intrinsic heart rate (ie, the rate independent of autonomic influences) is the sinus rate during pharmacologic denervation of the sinus node using a β-blocker and atropine. The intrinsic heart rate is sometimes used to distinguish healthy persons from those with sinus node dysfunction.
Sinus node recovery time is the interval between the end of a period of pacing-induced overdrive suppression of sinus node activity and the return of sinus node function, manifested on the surface ECG by the postpacing sinus P-wave interval. The measured SNRT depends on several factors, among them the proximity of the pacing catheter to the sinus node, the SACT, the presence or absence of SA entrance block (in which atrial impulses fail to enter and depolarize the sinus node), and local neurohormonal influences. In normal persons, atrial pacing at rates of 120–130 bpm for 30 seconds or more is followed by a return of sinus node activity at a reproducible interval, with the basic sinus rate generally being achieved within three postpacing beats. The usual SNRT is less than 1.5 seconds, although considerable variation may exist depending on the prevailing autonomic tone. The corrected sinus node recovery time can be calculated by subtracting the basic sinus rate from the sinus node recovery time; a normal value is usually between 350 and 550 ms. In patients with sinus node dysfunction, sinus node recovery times are not reproducible and tend to be longer after more prolonged periods of pacing; return to the basic sinus rate within three postpacing beats is also inconstant and may be followed by additional (secondary) pauses in rate.
SA conduction time reflects the time taken by a premature atrial-pacing stimulus delivered near the sinus node area to traverse the atrial tissue to reach the sinus node and prematurely depolarize it, the time to the formation of the next sinus impulse following the premature depolarization, and the return of the sinus-generated impulse through atrial tissue to the recording electrode. The SA conduction time is often prolonged in patients with clinical evidence of sinus node dysfunction other than sinus bradycardia alone.
Because the electrophysiologic tests of sinus node function are neither specific nor sensitive, they can sometimes show abnormal results in patients without sinus bradycardia and normal results in those with symptomatic sinus node disease. The test results should not, therefore, be relied on in isolation when making clinical decisions regarding either diagnosis or treatment.
Some of the problems of poor sensitivity and specificity can be overcome by recording the sinus node electrogram from an electrode catheter positioned in the vicinity of the sinus node (at the junction of the superior vena cava and the right atrium). The sinus node electrogram, however, cannot be reproducibly recorded in as many as 50% of patients. Sinus node electrography can distinguish between SA exit block and sinus arrest, and studies have shown that most pauses in sinus rhythm are due to SA exit block. Notwithstanding these technologic advances, the diagnosis of sinus node dysfunction generally remains a clinical one.
C. EXERCISE TESTING—Treadmill exercise testing can be of substantial value in assessing chronotropic response (“chronotropic competence”) to increases in metabolic needs in patients with sinus bradycardia in whom sinus node dysfunction is suspected. The definition of chronotropic incompetence is not agreed upon, but it is reasonable to designate it as consisting of either an inability to achieve a heart rate exceeding 75% of age-predicted maximum (usually taken as 220 – age), or 100–120 bpm at maximum effort. Irregular (and nonreproducible) increases, and even decreases, in sinus rate during exercise can also occur, but are rare. Similarly rare are abrupt changes in rate occurring during the postexercise recovery period. Chronotropic incompetence can result from medications (see Table 14–1) and should be distinguished from intrinsic sinus node dysfunction.
The Bruce treadmill exercise protocol, which is usually used to diagnose the presence and severity of coronary artery disease, is generally inappropriate for patients with sinus node dysfunction, in whom the goal is to assess heart rate at lower workloads expected to be encountered during average daily activities. Specific protocols, such as the chronotropic assessment exercise protocol (CAEP), have therefore been developed for this purpose. In addition to documenting chronotropic incompetence, treadmill exercise testing can be used to aid in optimal programming of rate-adaptive cardiac pacemakers that are usually required in these patients.
2. Atrioventricular block
A. ELECTROGRAPHY AND ELECTROCARDIOGRAPHY—The advent of intracardiac His bundle electrography has provided important information regarding normal and abnormal AV conduction in humans. The technique involves positioning a multipolar electrode catheter across the tricuspid valve in proximity to the AV nodal-His bundle area to record electrical activity as it passes through these structures. Because of its location, the catheter records electrical activity at the level of the low right atrium, His bundle, and proximal right bundle branch in addition to ventricular electrical activity. The sinus node pacemaker cells normally initiate the cardiac impulse, but they are not registered on either the surface ECG or the His bundle electrogram. The onset of the P wave on the surface ECG signifies the beginning of atrial depolarization; because the intracardiac electrode catheter lies at the level of the low right atrium, the early regions of atrial depolarization will not be detected by it; as the atrial depolarization wavefront passes through the region in which the catheter is located (the low right atrium), a deflection is registered (A). As the impulse traverses the His bundle, another deflection is registered, representing its depolarization (H). The His bundle deflection is followed by a ventricular deflection (V), which is registered at the time the wavefront of ventricular depolarization reaches the electrodes and often follows the onset of inscription of the QRS complex on the surface ECG.
His bundle electrography is useful in indicating the site of AV conduction delay or block. Normally, the conduction time through the AV node is 90–150 ms, and the conduction time through the His-Purkinje system is 35–55 ms. In a patient with a prolonged PR interval, a prolonged AH interval signifies delayed impulse conduction within the AV node, and a prolonged HV time represents delayed impulse conduction within the His bundle or the bundle branches. Conduction delay within the His bundle itself manifests as more than one His deflection (“split” His deflections).
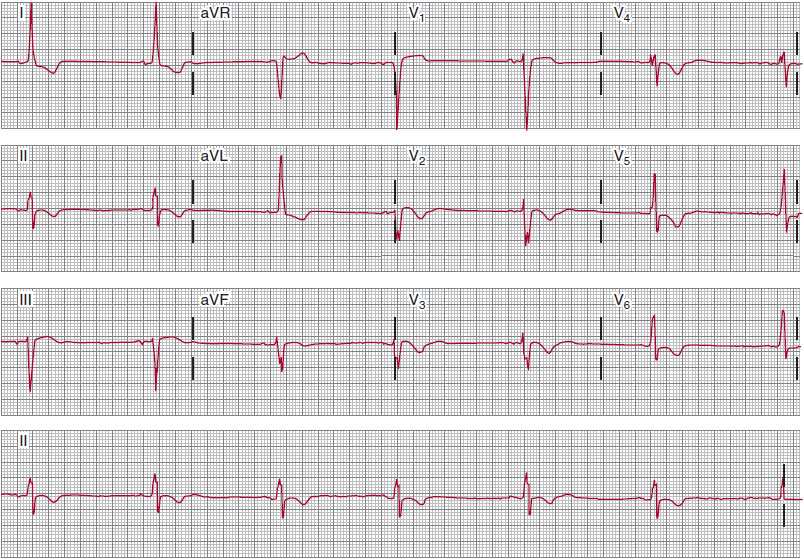
In first-degree AV block (a delay in conduction between the atria and the ventricles), all atrial impulses are conducted to the ventricles; it is characterized by a prolonged PR interval that exceeds 200 ms (Figure 14–8). The components of the PR interval are interatrial conduction (10–50 ms), AV nodal conduction (90–150 ms), and intra-His and His-Purkinje conduction (35–55 ms). The conduction delay in first-degree AV block can thus represent prolonged intraatrial, AV nodal, intra-His, or His-Purkinje conduction, and the recordings help clarify the location of the delay.
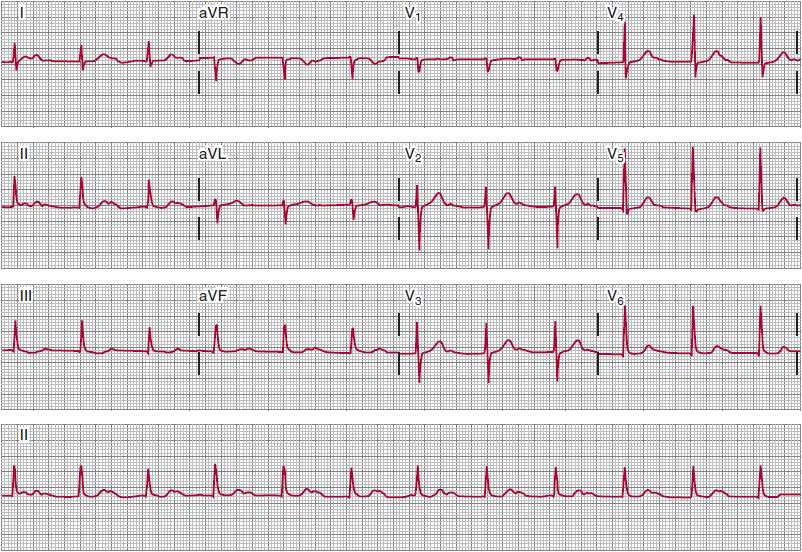
![]() Figure 14–8. Sinus rhythm with marked first-degree atrioventricular (AV) block. All P waves are conducted to the ventricles. The PR intervals are about 480 ms. The RP intervals are shorter than the PR intervals, which, in some patients, can cause symptoms due to suboptimally timed AV depolarization-contraction sequences. Despite the length of the PR intervals, this conduction disturbance is generally benign; evolution to second-degree AV block can take years.
Figure 14–8. Sinus rhythm with marked first-degree atrioventricular (AV) block. All P waves are conducted to the ventricles. The PR intervals are about 480 ms. The RP intervals are shorter than the PR intervals, which, in some patients, can cause symptoms due to suboptimally timed AV depolarization-contraction sequences. Despite the length of the PR intervals, this conduction disturbance is generally benign; evolution to second-degree AV block can take years.
In patients with a QRS complex that is narrow and normal-appearing, first-degree AV block is AV nodal in more than 85% and is intra-His in less than 15%. In patients with a wide QRS complex, first-degree AV block is AV nodal in less than 25%, infranodal in about 45%, and at more than one site in about 33%.
In second-degree AV block, not all atrial impulses are conducted to the ventricles. The ratio of P waves to QRS complexes describes the AV conduction ratio. Type I (Wenckebach) second-degree AV block is present when the conduction of atrial impulses to the ventricles is progressively delayed because of AV (generally AV nodal) refractoriness, with eventual failure of conduction of an atrial impulse to the ventricles. The AV conduction ratio in type I second-degree AV block can be 3:2, 4:3, 5:4, and so on; this ratio is also referred to as a Wenckebach period. AV conduction ratios in type I second-degree AV block need not be constant, and therefore, the Wenckebach period may not be reproducible. Because type I second-degree AV block usually occurs within the AV node, the PR interval of the first conducted P wave of the Wenckebach period is often prolonged, and because this conduction disturbance does not involve the bundle branches, the QRS complexes are expected to be narrow and normal-appearing unless there is preexisting bundle branch disease.
In a typical, or classic Wenckebach period, the PR intervals progressively lengthen, the R-R intervals progressively shorten, and the R-R interval encompassing the nonconducted P wave is less than twice the preceding R-R interval. Typical Wenckebach periods are usually seen with low AV conduction ratios (3:2, 4:3, and 5:4), but as the AV conduction ratio increases (exceeding 6:5), more and more Wenckebach sequences are atypical and do not follow the rules. If the sinus rate is not constant, for example in vagal bradycardias, sequences that resemble Wenckebach conduction (referred to as “vagotonic block”) often occur; they should, however, not be considered type I second-degree AV block, in which the sinus rate must be constant for the diagnosis to be made.
In type II second-degree AV block, atrial impulses intermittently fail to be transmitted to the ventricles, but progressive conduction delay prior to the conduction failure does not occur. Because prior conduction delay from the atria is not present, the failure of antegrade conduction is often abrupt and unpredictable and may be advanced. In contrast to type I second-degree AV block, in which the conduction delay is usually in the AV node, the conduction delay in type II second-degree AV block can be within the His bundle or, more commonly, distal to the His bundle in the bundle branches. If the block is within the His bundle, the QRS complexes will be narrow and normal-appearing, or only mildly aberrant, unless preexisting BBB is present. If the block is infra-His, the QRS complexes will show a BBB pattern. In contrast to type I second-degree AV block, the PR interval of the conducted P waves is constant and often (but not always) normal (Figure 14–9).
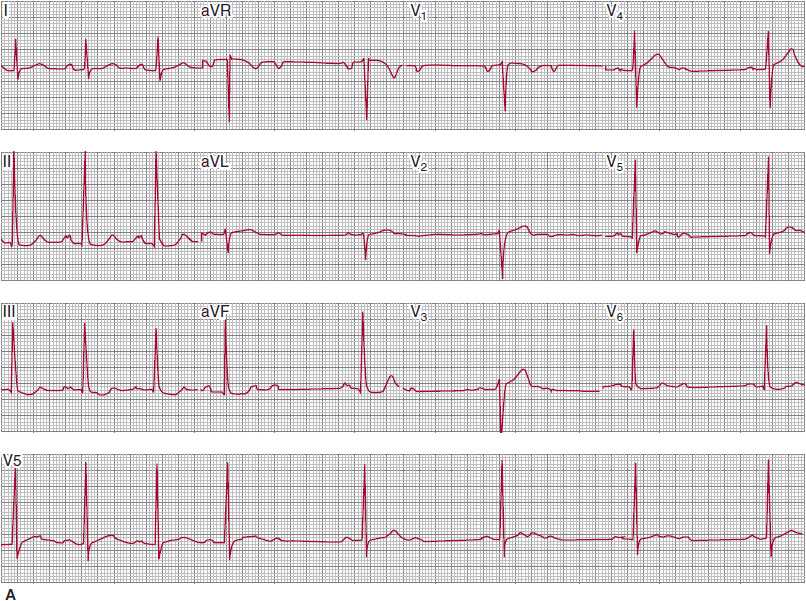
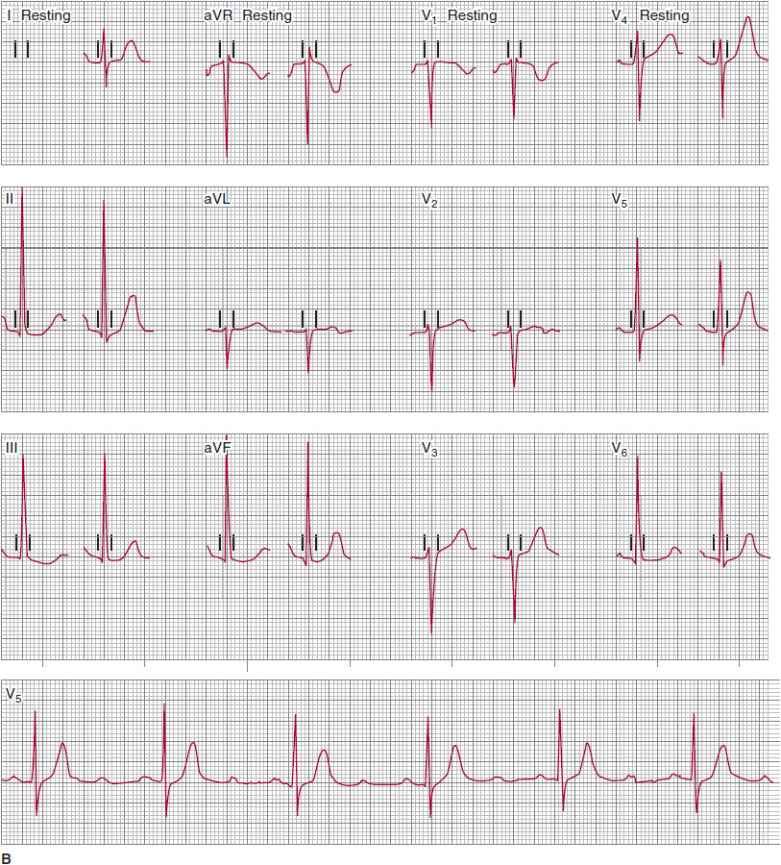
![]() Figure 14–9. A: This electrocardiogram was recorded in a patient with presyncopal spells who was about to undergo exercise treadmill testing. The atrial rhythm is sinus at a rate of about 68 bpm. The PR intervals of the conducted QRS complexes are all about 240 ms. The QRS complexes are narrow, and nondiagnostic ST- and T-wave abnormalities are present. A 2:1 atrioventricular (AV) conduction develops abruptly during the recording. The prolonged PR intervals of the conducted P waves, as well as the narrow morphology of the QRS complexes (indicating absence of bundle branch system disease), could suggest that the 2:1 AV conduction represents AV nodal block; intra-His block, however, is suggested by the absence of prolongation of the PR intervals prior to the nonconducted P waves. Changing of the AV conduction ratio from 2:1 to 3:2, 5:4, and so on, would help establish the presence of AV nodal block. This could be achieved by atropine or exercise testing, during which adrenergic drive would be expected to facilitate AV conduction. B: The patient achieved stage IV of the Bruce protocol during treadmill testing. The atrial rate was 150 bpm. At peak effort and for the first 2 minutes of the postexercise recovery period, 3:1 atrioventricular (AV) block developed abruptly and was associated with the patient’s typical presyncopal symptoms. AV block developing during exercise, although decidedly rare, is always abnormal, and if the QRS complexes are narrow or normal appearing, indicate intra-His block. Permanent cardiac pacing is required.
Figure 14–9. A: This electrocardiogram was recorded in a patient with presyncopal spells who was about to undergo exercise treadmill testing. The atrial rhythm is sinus at a rate of about 68 bpm. The PR intervals of the conducted QRS complexes are all about 240 ms. The QRS complexes are narrow, and nondiagnostic ST- and T-wave abnormalities are present. A 2:1 atrioventricular (AV) conduction develops abruptly during the recording. The prolonged PR intervals of the conducted P waves, as well as the narrow morphology of the QRS complexes (indicating absence of bundle branch system disease), could suggest that the 2:1 AV conduction represents AV nodal block; intra-His block, however, is suggested by the absence of prolongation of the PR intervals prior to the nonconducted P waves. Changing of the AV conduction ratio from 2:1 to 3:2, 5:4, and so on, would help establish the presence of AV nodal block. This could be achieved by atropine or exercise testing, during which adrenergic drive would be expected to facilitate AV conduction. B: The patient achieved stage IV of the Bruce protocol during treadmill testing. The atrial rate was 150 bpm. At peak effort and for the first 2 minutes of the postexercise recovery period, 3:1 atrioventricular (AV) block developed abruptly and was associated with the patient’s typical presyncopal symptoms. AV block developing during exercise, although decidedly rare, is always abnormal, and if the QRS complexes are narrow or normal appearing, indicate intra-His block. Permanent cardiac pacing is required.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


