The aim of this study is to evaluate the safety and feasibility of using the Amigo Remote Catheter System (RCS) in arrhythmia ablation procedures. Because Amigo allows the physician to operate all catheter function outside of the radiation field, operator exposure time was also evaluated. This is a nonrandomized, prospective clinical trial conducted at 1 site (identifier: NCT01834872 ). The study prospectively enrolled 50 consecutive patients (mean age 59 ± 15 years, 72% men) with any type of arrhythmia (23 atrial fibrillation ablation, 12 common atrial flutters, 10 patients with other supraventricular tachycardia, 4 ventricular tachycardia, and 1 patient with palpitations with no arrhythmia induced) referred for catheter ablation, in which we used RCS. Fifty matched ablation procedures (mean age 57 ± 14 years, 70% men) performed during the same time period, without RCS, were enrolled into the control group. Acute ablation success was 96% with RCS and 98% in the manual group. In only 2 cases, the physician switched to manual ablation (1 ventricular tachycardia and 1 accessory pathway) to complete the procedure. There were no complications related to the use of RCS. No differences were observed in total procedure time, total fluoroscopy time, or total radiofrequency delivery compared with the manual group. In procedures performed with RCS, the operator’s fluoroscopy exposure time was reduced by 68 ± 16%. In conclusion, arrhythmia ablation with RCS is safe and feasible. Furthermore, it significantly reduces operator’s exposure to radiation.
Conventional radiofrequency ablation of cardiac arrhythmias has emerged as a standard therapy with high success and low complication rates. Remote navigation systems are commercially available with the aim to reduce x-ray exposure while maintaining or improving patient safety, procedure times, and ablation success rates. The Amigo Remote Catheter System (RCS; Catheter Robotics Inc., Mount Olive, New Jersey) is a new system that has the benefit of using standard commercially available catheters. The aim of this prospective observational study was to evaluate the performance of Amigo RCS in ablation procedures for most common arrhythmias compared with a conventional manual approach.
Methods
The study protocol was approved by the ethical committee and has been registered in ClinicalTrials.gov (identifier: NCT01834872 ). All patients provided written informed consent before inclusion.
We prospectively enrolled 50 consecutive patients referred to our electrophysiology (EP) laboratory to treat any type of arrhythmia with catheter ablation, in which we used Amigo RCS. All procedures were performed by 2 operators. As a control group, patients were prospectively recruited by matched ablation procedures performed by the same operators during the same period of time. Cases were matched by substrate and procedure type resulting in a “treatment group” (with RCS, n = 50) and a “control group” (without RCS, n = 50). Data from both RCS and conventional approach were collected prospectively from September 24, 2012 to March 19, 2013. Study investigators had previous experience with the use of Amigo. Before this clinical trial, the 2 operators performed 36 procedures with the Amigo RCS, most of which were right-side procedures.
The Amigo RCS consists of a robotic arm installed on the patient table and a remote controller connected to the arm through a cable ( Figure 1 ). Amigo does not incorporate separate workstations, monitors, or other hardware. Catheter visualization is done using standard fluoroscopic techniques. Amigo attaches to the rails of a standard EP table; the system can be transported from one room to another within minutes. Amigo (Catheter Robotics Inc., Mount Olive, New Jersey) has CE mark approval for use with Boston Scientific Blazer and Biosense Webster EZ Steer catheters. The catheter is placed on the docking station and is manipulated by the controller, which may be up to 30 m away from the patient. This allows the operator to control catheter navigation outside of the fluoroscopy field.
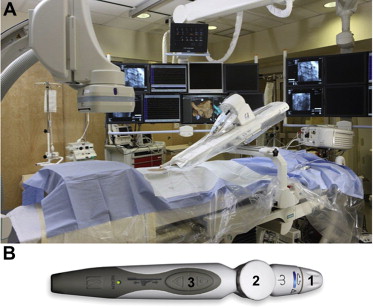
The remote controller mimics a bidirectional EP catheter handle by mechanically controlling the catheter just as a physician would do manually, allowing the physician to insert, withdraw, rotate the catheter, and deflect the catheter tip. As in a manual procedure, only the catheter and introducer are inside the patient’s body. The distal tip of the nosecone attaches to the hub of a standard introducer sheath.
Amigo uses sterile components that maintain the sterile field of the system and the catheter. If desired, the catheter can be removed from Amigo and the physician can assume manual manipulation without breaking the sterile field. When manual manipulation is finished, the sterile catheter can be returned to the Amigo system and remote manipulation resumed.
After providing written informed consent, each patient underwent an electrophysiological study and catheter ablation while in the fasting nonsedated state. The same standard accepted approach was used for any type of arrhythmia in both groups. When using RCS, after obtaining the vascular access and positioning diagnostic catheters, the ablation catheter was positioned on the RCS arm, and from this moment the physician operated outside the radiation area. At any time, the physician could switch to manual manipulation of the roving ablation catheter.
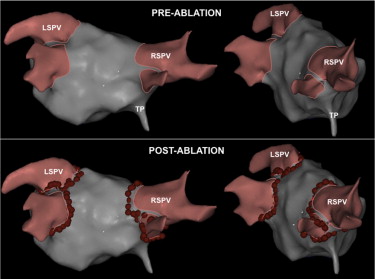
In left ventricular tachycardias (VTs), a retrograde approach through the aortic valve was used. The roving catheter was attached to the RCS before or after crossing the aortic valve, at the discretion of the attending physician. In atrial fibrillation (AF) ablation cases, the ablation catheter (irrigated-tip 3.5-mm ablation catheter) was connected to the robotic arm after a successful transseptal procedure. A double transseptal access through a single puncture site was used. A long sheath was used to introduce only the circular catheter, not the ablation catheter. Thus, the robotic arm was connected to an introducer, as in the other procedures. Continuous circumferential lesions were created with an irrigated-tip ablation catheter encircling the right and left pulmonary vein (PV) ostia individually and guided by the Carto 3 (Biosense Webster, LTD; Haifa, Israel) navigation system, which was operated outside the radiation area ( Figure 2 ). After completion of the circumferential lesion set, the ipsilateral superior and inferior PVs were mapped by a circular catheter (Lasso; Biosense Webster). If PVs were still connected to the left atrium, supplementary ablation applications were applied along the circumferential lines to electrically isolate PVs. PV isolation was considered successful when bidirectional PV-left atrial conduction block was achieved. Other ablation lines could be performed at the discretion of the attending physician.
The following prespecified procedure variables were analyzed: acute ablation success (the acute end point of each procedure, indicated in the Supplementary material ); proportion of cases in which physician switched from RCS to manual ablation; procedure acute complications; procedure time (from vein or artery access to introducer sheath removal); radiofrequency delivery time; fluoroscopy time; and operator’s fluoroscopy exposure time.
All statistical analyses were performed using SPSS software, version 16.0 (SPSS Inc., Chicago, Illinois). All continuous data are presented as mean ± SD. Categorical variables were compared using chi-square test or with Fisher’s exact test if the sample size was <5 in a given cell. Comparisons of continuous data were performed with Student t test. All the data satisfied criteria for normality of distribution (Kolmogorov-Smirnov). Two-sided p value <0.05 was considered significant.
Results
Patient characteristics ( Table 1 ) were similar in both groups. Fifty percent of patients in both groups were left-sided procedures. AF was paroxysmal in 13 patients (57%) of RCS group and in 11 controls (47%). There were 4 VTs: 2 idiopathic, 1 with ischemic cardiomyopathy, and 1 with dilated cardiomyopathy (identical in both groups). The type of ablation catheter that was used in each arrhythmia substrate is shown in the Supplementary material . In the RCS group, a Biosense Webster EZ Steer was used in 34 procedures and a Boston Scientific Blazer in 16 (8 cases with 4 mm, 7 with 8 mm, and 1 with an open-irrigated catheter).
| Variable | RCS (n = 50) | Controls (n = 50) | p Value |
|---|---|---|---|
| Age (yrs) | 59 ± 15 | 57 ± 14 | 0.504 |
| Men | 36 (72) | 35 (70) | 0.826 |
| Body mass index (kg/m 2 ) | 27 ± 3 | 27 ± 4 | 0.961 |
| Diabetes mellitus | 6 (12) | 4 (8) | 0.741 |
| Hypertension | 20 (40) | 15 (31) | 0.329 |
| Left ventricular ejection fraction (%) | 57 ± 13 | 57 ± 14 | 0.982 |
| Cardiomyopathy | 10 (20) | 11 (22) | 0.806 |
| Previous heart surgery | 3 (6) | 2 (4) | 1.00 |
| Arrhythmia type | 1.00 | ||
| AF | 23 (46) | 23 (46) | |
| Common atrial flutter | 12 (24) | 12 (24) | |
| Atrioventricular node reentrant tachycardia | 5 (10) | 5 (10) | |
| Accessory pathway | 3 (6) | 3 (6) | |
| Atypical atrial flutter/atrial tachycardia | 2 (4) | 2 (4) | |
| VT | 4 (8) | 4 (8) | |
| Palpitations (none diagnosis) | 1 (2) | 1 (2) |
Two patients in each group were excluded from the acute success analysis due to the ablation procedures not being performed (n = 48 in each group for these analysis). In 2 patients (1 in each group) whose indication for the procedure was palpitations, no tachycardia was induced during the EP study, so ablation was not necessary. One patient in the RCS group had a focal right atrial tachycardia. The tachycardia originated in the crista terminalis, where the right phrenic nerve was captured ( Figure 2 ). As the tachycardia was well tolerated by the patient, the physician chose not to ablate and to treat this arrhythmia medically. The remaining excluded case was a patient in the manual group with a focal left VT that originated close to the His bundle, in which ablation was not attempted.
Acute ablation success was achieved in 46 of 48 patients (96%) in the RCS group using only the RCS. This included 23 cases of AF ablation, in which all PVs were isolated. In only 2 cases, the physician started with RCS, but switched to manual manipulation of the catheter (these procedures were considered as failures for the present analysis). The first patient had a parahissian accessory pathway and the physician decided to use cryotherapy to ablate it. Cryoablation catheters are not compatible with the Amigo RCS, thus manual manipulation of the catheter was required and cryoablation effectively eliminated the accessory pathway. The second case was a left-sided VT. The patient had a bicuspid aortic valve that was difficult to cross, so the physician chose to change to manual handling. The tachycardia was effectively ablated. Acute ablation success was achieved in 47 of 48 patients in the control group (98% acute success rate). In 1 common atrial flutter case, cavotricuspid isthmus block could not be achieved.
There were no complications secondary to the use of the robotic system. Only 2 patients experienced acute complications, 1 in each group. One patient in the RCS group with moderate left ventricular dysfunction developed severe hypotension after being anesthetized during an AF ablation procedure. He recovered with dobutamine, and the procedure was performed without further intervention. One patient in the control group had ventricular dysfunction (ejection fraction of 45%), and developed pulmonary edema after AF ablation. Radiofrequency delivery time (39 minutes) and procedure duration (190 minutes) were consistent with that of a typical AF ablation procedure. The patient received medical treatment and recovered without further problems.
We did not observe differences in procedure parameters between groups, neither in the whole population ( Table 2 ) nor by arrhythmia substrate ( Table 3 ). However, using the Amigo RCS, the average radiation exposure time of the operator was significantly reduced by 68% (from 41 to 13 minutes).
| Variable | RCS (n = 50) | Controls (n = 50) | p Value |
|---|---|---|---|
| Procedure time (minutes) | 151 ± 59 | 148 ± 62 | 0.851 |
| Radiofrequency delivery time (minutes) | 26 ± 18 | 25 ± 15 | 0.762 |
| Fluoroscopy time (minutes) | 41 ± 18 | 42 ± 19 | 0.789 |
| Fluoroscopy dose (mGy/m 2 ) | 20,501 ± 15,146 | 23,937 ± 15,733 | 0.269 |
| Fluoroscopy exposure (minutes) | 13 ± 11 | 42 ± 19 | <0.0001 |
| Irrigated-tip catheter | 35 (70) | 31 (62) | 0.398 |
| 3D navigator system | 32 (64) | 30 (60) | 0.680 |
| Acute ablation success ∗ | 46 (96) | 47 (98) | 1.00 |
| Acute complications | 1 (2) | 1 (2) | 1.00 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


