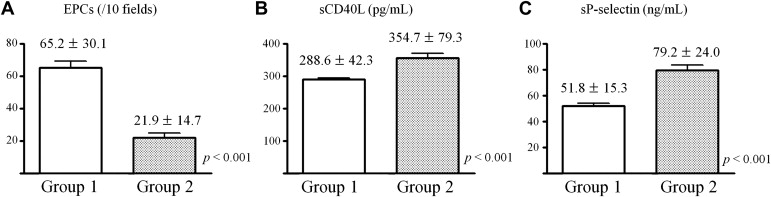
Although high on-treatment platelet reactivity (HTPR) is an important predictor of clinical outcomes in patients undergoing coronary stenting, it is unknown whether endothelial dysfunction and HTPR are associated. We examined the platelet function, peripheral vascular function, endothelial progenitor cell (EPC) number, platelet activation markers, high-sensitivity C-reactive protein (hs-CRP) level, and clinical outcomes in patients receiving chronic clopidogrel therapy. We consecutively enrolled 91 patients who underwent follow-up angiography because of chest discomfort. All patients took aspirin and clopidogrel for an average of 498 ± 138 days. Platelet reactivity was assessed by light transmittance aggregometry (maximal platelet aggregation by 5 μmol/L of adenosine diphosphate ≤50% in group 1 [optimal response] and >50% as group 2 [HTPR]). Flow-mediated dilation of the brachial artery and brachial-ankle pulse wave velocity (PWV), numbers of EPCs isolated from peripheral blood, platelet activation markers (soluble CD40 ligand and soluble P-selectin), and hs-CRP levels were assessed before follow-up angiography. There were no significant differences in baseline characteristics and previous percutaneous coronary intervention (PCI) data between groups 1 (n = 59) and 2 (n = 32). Group 2 showed poorer flow-mediated dilation (6.1 ± 4.1% vs 12.9 ± 6.2%, p <0.001), pulse wave velocity (1925.4 ± 362.2 vs 1571.0 ± 306.5 ms, p <0.001), and lower circulating EPCs by flow cytometry (21.9 ± 14.7 vs 65.2 ± 30.1 per 10 fields, p <0.001) compared with group 1. Significantly higher levels of soluble CD40 ligand, soluble P-selectin, and hs-CRP were observed in group 2. In multivariate analysis, elevated hs-CRP level, but not HTPR, was independently associated with repeated PCI. In patients with angina, HTPR was associated endothelial dysfunction and elevated hs-CRP, although elevated hs-CRP level was significantly associated with poorer outcomes.
Cumulative evidence has demonstrated the poor prognostic value of high on-treatment platelet reactivity (HTPR) during aspirin and clopidogrel therapy in patients undergoing coronary stenting. However, these exacerbated clinical outcomes in patients with HTPR would not solely depend on persistent platelet activation. A better understanding of the pleiotropic effects of clopidogrel would clarify the contribution of proinflammatory mediators and endothelial function impairment to high-risk atherosclerotic complications in patients with HTPR. High platelet reactivity soon after initiation of dual antiplatelet therapy with aspirin and clopidogrel could be improved with longer treatment ; thus, we investigated whether high platelet reactivity determined during a steady-state phase of dual antiplatelet therapy was associated with endothelial dysfunction, circulating endothelial progenitor cell (EPC) numbers, platelet activation markers, and high-sensitivity C-reactive protein (hs-CRP) in patients with stable angina pectoris.
Methods
Patients admitted to the hospital (n = 204) for a follow-up coronary angiography because of recurrent chest discomfort from September 2009 to August 2010 were eligible for this study. All patients had previously undergone successful percutaneous coronary intervention (PCI) with at least 1 drug-eluting stent for stable angina and received 100-mg aspirin and 75-mg clopidogrel therapy for at least 6 months. Patients were excluded from the study if they had previously undergone PCI because of in-stent restenosis or ST elevation myocardial infarction. The other exclusion criteria were a platelet count of <1 × 10 6 /μl; hematocrit <25%; liver disease (bilirubin >2 mg/dl); active bleeding or bleeding diathesis; hemodynamic instability; acute coronary or cerebrovascular event within 3 months; malignancy; concomitant use of oral anticoagulants, dipyridamole, oral glucocorticoids, or a nonsteroidal antiinflammatory drug; and recent treatment (<30 days) with a glycoprotein IIb/IIIa antagonist.
All vasoactive medications, except aspirin and clopidogrel, were discontinued for ≥24 hours before blood sample withdrawal for EPC count, hs-CRP, and platelet reactivity assessments. A peripheral vascular evaluation (flow-mediated dilation [FMD] of the brachial artery and brachial-ankle pulse wave velocity [PWV]) was performed between 8 a.m. and 10 a.m. in a quiet temperature-controlled room (22°C to 24°C) after a 12-hour fast and before coronary angiography. After angiography, repeated PCI was performed if indicated. The study protocol was approved by the Institutional Clinical Research and Ethics Committee of Kyung Hee University and all patients gave their informed consent in writing.
Platelet function was assessed with platelet-rich plasma by the turbidimetric method using 2-channel aggregometry with a Chrono-Log 490 Model (Chrono-log Corp., Havertown, Pennsylvania) as described previously. Platelet function was measured after addition of 5-μmol/L adenosine diphosphate (ADP), and curves were recorded for 7 minutes. The HTPR was defined as maximal platelet aggregation by 5 μmol/L of ADP >50% based on previous studies. Optimal clopidogrel response was defined as platelet aggregation by 5 μmol/L of ADP ≤50%.
Before coronary angiography, quantitative measurements of hs-CRP levels with routine laboratory tests were performed. Additionally, the markers of platelet activation (soluble CD40 ligand and soluble P-selectin) were assessed using a quantitative enzyme-linked immunosorbent assay technique (human soluble CD40 ligand [BMS239] and human soluble P-selectin [BMS219/3]; Bender MedSystems GmbH, Vienna, Austria) according to the manufacturer’s instructions. Whole blood was centrifuged for 15 minutes at 3,000 rpm, aliquoted, and immediately frozen at −80°C until analysis. All samples were run in the same assay and all assays were performed in duplicate.
Based on cell surface antigen expression, circulating mononuclear cells with CD34 + CD133 + or CD34 + CD133 + VEGFR2 + were quantified as tentative PCs or EPCs. White blood cells were stained with allophycocyanin-conjugated anti-CD45 monoclonal antibody, fluorescein isothiocyanate–conjugated anti-CD34 monoclonal antibody, and phycoerythrin-conjugated anti-VEGFR2 monoclonal antibody, and further incubated in the dark for 20 minutes. After appropriate gating based on low cytoplasmic granularity and low expression of CD45, CD34 + VEGFR2 + cells were enumerated and expressed as the number of cells/10 6 total events or number of cells/100 μl of blood.
FMD and PWVs were assessed by the same examiner, who was unaware of the subjects’ clinical backgrounds. Brachial artery FMD was examined using a commercially available system (Vivid 7; GE Vingmed, Horten, Norway) equipped with a 14-MHz linear array transducer as we described previously. FMD was calculated as the percentage maximum increase in arterial diameter. Brachial artery diameter was calculated from the trailing edge of the intima-blood interface to the leading edge semiautomatically using a modified version of the ImageJ software (National Institutes of Health, Bethesda, Maryland), as well as custom-designed software. PWVs were measured using an automated PWV/ankle-brachial index device (Colin Co. Ltd., Komaki, Japan) after FMD measurements with the subject in the supine position. The details of the measurement, validity, and reproducibility of this method have been reported previously. The average of left and right brachial-ankle PWV in each subject was used for the analysis.
Coronary angiography and repeat PCI, if indicated, were performed using standard techniques. Angiographic in-stent restenosis was defined as >50% luminal narrowing at the segment site, including the stent and 5 mm proximal and distal to the stent edges of the target vessel, on the follow-up angiogram. A de novo lesion was defined as >70% stenosis in at least 1 major coronary artery. The number of diseased coronary arteries was also recorded.
The hypothesis of the study was that patients with HTPR after chronic clopidogrel administration would demonstrate impaired endothelial function compared with patients with optimal platelet reactivity. We determined the sample size calculation from the previously reported incidence of HTPR (30%) in our patient population. Therefore, to detect a statistically significant difference with a power of 80% with a 2-sided α level of 0.05, a sample of 91 patients would be required to detect a 40% difference in the FMD between patients with optimal platelet reactivity and those with HTPR.
The analyses were performed using SPSS, version 17.0 (SPSS, Chicago, Illinois). Differences were considered significant if the p value using a 2-sided test was <0.05. Continuous variables, presented as means ± SD, were evaluated for normal distribution and compared using the Student t test or Mann-Whitney test accordingly. The continuous parameters with a skewed distribution were logarithmically transformed. Categorical variables, presented as frequencies and percentages, were compared using the chi-square test or Fisher’s exact test as appropriate. Correlations between 2 continuous variables were performed using the Pearson correlation coefficient or Spearman’s rank correlation, if not normally distributed. The cumulative de novo lesion progression and repeated PCI rates were drawn using the Kaplan-Meier method and the log-rank test was used to compare survival between the 2 groups. A Cox proportional hazards model was used for the multivariate analysis (forward stepwise) to determine which parameters identified using univariate analysis were significantly associated with ischemic cardiac events.
Results
Figure 1 depicts the study design. After the exclusion of patients who did not meet enrollment criteria, 91 patients completed the study protocol. The mean time from previous PCI to follow-up coronary angiography was 498 ± 138 days. Of the 91 patients, 59 showed an optimal clopidogrel response (group 1) and 32 patients showed HTPR (group 2). Baseline characteristics of each group are listed in Table 1 . There were no significant differences in demographic characteristics, cardiovascular risk factors, and concomitant medications. To explore the correlations of medications and laboratory tests, the types of statins and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were also evaluated. The type of statin prescribed did not impact hs-CRP, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers did not affect EPC counts (data not shown).
| Variable | Group 1 (n = 59) | Group 2 (n = 32) | p Value |
|---|---|---|---|
| Age (yrs) | 57 ± 9 | 56 ± 11 | 0.29 |
| Men | 37 (63) | 21 (65) | 0.78 |
| Weight (kg) | 65 ± 9 | 68 ± 10 | 0.84 |
| Body mass index (kg/m 2 ) | 25 ± 3 | 25 ± 3 | 0.91 |
| Systolic blood pressure (mm Hg) | 122 ± 13 | 120 ± 13 | 0.52 |
| Diastolic blood pressure (mm Hg) | 77 ± 8 | 75 ± 10 | 0.24 |
| Heart rate (beats/min) | 73 ± 14 | 74 ± 9 | 0.93 |
| Hypertension ∗ | 47 (80) | 29 (90) | 0.18 |
| Diabetes mellitus | 9 (15) | 7 (22) | 0.43 |
| Smoker | 18 (30) | 12 (37) | 0.49 |
| Dyslipidemia † | 45 (76) | 24 (75) | 0.89 |
| Glucose (mg/dl) | 160 ± 50 | 166 ± 60 | 0.19 |
| Total cholesterol (mg/dl) | 184 ± 37 | 183 ± 42 | 0.37 |
| Triglyceride (mg/dl) | 126 ± 63 | 130 ± 68 | 0.23 |
| Low-density lipoprotein cholesterol (mg/dl) | 124 ± 43 | 136 ± 77 | 0.18 |
| High-density lipoprotein cholesterol (mg/dl) | 48 ± 12 | 44 ± 11 | 0.78 |
| Angiotensin-converting enzyme inhibitor | 6 (10) | 4 (12) | 0.74 |
| Angiotensin receptor blocker | 39 (66) | 23 (72) | 0.57 |
| β Blocker | 39 (66) | 25 (78) | 0.23 |
| Calcium channel blocker | 8 (13) | 4 (12) | 0.89 |
| Nitrate | 23 (39) | 14 (44) | 0.82 |
| Oral hypoglycemic agent | 6 (10) | 5 (15) | 0.45 |
| Insulin | 3 (5) | 2 (6) | 0.82 |
| Statins | 45 (76) | 24 (75) | 0.89 |
∗ Systolic pressure >140 mm Hg and diastolic pressure >90 mm Hg or use of antihypertensive medications.
† Total cholesterol >200 mg/dl, low-density lipoprotein >130 mg/dl, or use of cholesterol-lowering medications.
Group 2 had significantly less EPCs and higher platelet activation markers (plasma soluble CD40 ligand and soluble P-selectin) compared with group 1 ( Figure 2 ). As shown in Figure 3 , hs-CRP was also higher in group 2 and there was a positive correlation between hs-CRP and platelet reactivity. As presented in Table 2 and Figure 4 , group 2 showed significantly less FMD and more PWVs compared with group 1. Maximal platelet aggregation was negatively correlated with FMD and positively correlated with PWVs. Similar differences in biomarkers and correlations were obtained when 20-μmol/L ADP-induced aggregation max >60% (higher tertile) was used to define HTPR (data not shown).

| Variable | Group 1 (n = 59) | Group 2 (n = 32) | p Value |
|---|---|---|---|
| Baseline brachial artery diameter (mm) | 4.2 ± 0.6 | 4.2 ± 0.7 | 0.69 |
| Baseline blood flow (cm/s) | 24.8 ± 5.9 | 29.1 ± 9.2 | 0.19 |
| Diameter after reactive hyperemia (mm) | 4.7 ± 0.6 | 4.5 ± 0.7 | 0.13 |
| Flow after reactive hyperemia (cm/s) | 76.9 ± 8.5 | 80.9 ± 9.4 | 0.14 |
| FMD (%) | 12.9 ± 6.2 | 6.1 ± 4.1 | <0.01 |
Table 3 lists the previous PCI and follow-up angiography data of both groups. There were no significant differences in disease severity and deployed stent type. No cardiac death, repeated myocardial infarction, or stroke occurred. However, higher de novo lesion progression rates were noted in group 2 compared with group 1 after follow-up coronary angiography. Repeated PCI rates were marginal with no significant differences between groups 1 and 2 (p = 0.07). Multivariate Cox analysis determined that smoking and hs-CRP levels were independent predictors of de novo lesion progression ( Table 4 ). Rates of repeated PCI were associated significantly with hs-CRP levels.
| Variable | Group 1 (n = 59) | Group 2 (n = 32) | p Value |
|---|---|---|---|
| Previous PCI | |||
| Number of narrowed coronary arteries | 0.96 | ||
| 1 | 35 (59) | 18 (56) | |
| 2 | 14 (24) | 8 (25) | |
| 3 | 10 (17) | 6 (19) | |
| Type B2/C lesion | 32 (54) | 18 (56) | 0.85 |
| Stent type | 0.82 | ||
| Sirolimus eluting | 36 (61) | 19 (59) | |
| Paclitaxel eluting | 18 (31) | 9 (28) | |
| Zotarolimus eluting | 5 (8) | 4 (15) | |
| Total numbers of stents | 2.1 ± 1.2 | 2.3 ± 1.3 | 0.49 |
| Times from previous coronary intervention to follow-up coronary angiography (days) | 508 ± 146 | 478 ± 121 | 0.33 |
| Follow-up coronary angiography | <0.05 | ||
| No interval change | 48 (81) | 20 (62) | 0.07 |
| Repeated PCI | 11 (19) | 12 (38) | 0.07 |
| In-stent restenosis | 8 (14) | 5 (16) | 0.76 |
| De novo lesion | 3 (5) | 7 (22) | <0.05 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


