Patients with acute coronary syndrome are recommended for early aggressive low-density lipoprotein (LDL) cholesterol–lowering therapy. The LUNAR study compared the efficacy of rosuvastatin with that of atorvastatin in decreasing LDL cholesterol in patients with acute coronary syndrome. Adult patients with coronary artery disease who were hospitalized for an acute coronary syndrome within 48 hours of first symptoms were randomized (n = 825) to an open-label, once-daily treatment with rosuvastatin 20 mg (RSV20), rosuvastatin 40 mg (RSV40), or atorvastatin 80 mg (ATV80) for 12 weeks. Patients were evaluated at weeks 2, 6, and 12. The primary end point was treatment efficacy in lowering LDL cholesterol averaged over 6 to 12 weeks. Changes in other lipoproteins, including high-density lipoprotein (HDL) cholesterol, and safety were evaluated. Analysis of covariance was used to compare least squares mean differences between each rosuvastatin treatment arm and the atorvastatin arm. The efficacy of RSV40 in lowering LDL cholesterol was significantly greater than that of ATV80 (46.8% vs 42.7% decrease, p = 0.02). LDL cholesterol lowering by RSV20 was similar to that by ATV80. Increases in HDL cholesterol were significantly greater with RSV40 (11.9%, p <0.001) and RSV20 (9.7%, p <0.01) than with ATV80 (5.6%). RSV40 was also significantly more effective than ATV80 in improving most other secondary efficacy variables, whereas the effects of RSV20 on these parameters were generally similar to those of ATV80. All 3 treatments were generally well tolerated over 12 weeks. In conclusion, results from the LUNAR study show that RSV40 more effectively decreased LDL cholesterol, increased HDL cholesterol, and improved other blood lipid parameters than ATV80 in patients with acute coronary syndrome.
Acute coronary syndrome is associated with an increased risk of cardiovascular mortality and recurrent cardiac events, underscoring the importance of identifying treatments that minimize this risk. The Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) trial suggested a benefit of lipid lowering with high-dose atorvastatin in patients with acute coronary syndrome. Timing of statin administration and extent of lipid lowering in relation to onset of benefit on clinical outcomes and potential mechanisms by which statins are thought to be of benefit in patients with acute coronary syndrome are, however, controversial. A meta-analysis of statin use in patients with acute coronary syndrome confirmed the benefits of early high-dose statin administration in decreasing recurrent myocardial ischemia. In this analysis, the clinical benefit and safety of statins were suggested to be dose and statin dependent. Although there have been considerable data comparing the effectiveness of various statins, including atorvastatin 80 mg/day (ATV80) with rosuvastatin 20 mg/day (RSV20) and rosuvastatin 40 mg/day (RSV40), overall there has not been a systematic comparison of their lipid-lowering effects in patients with acute coronary syndrome. The Limiting Undertreatment of Lipids in Acute Coronary Syndrome with Rosuvastatin (LUNAR) study was therefore initiated to compare the efficacy of once-daily regimens of RSV20 and RSV40 with ATV80 in decreasing low-density lipoprotein (LDL) cholesterol levels in patients with acute coronary syndrome.
Methods
The study was approved by the local institutional review board/independent ethics committee and conducted in accordance with principles of good clinical practice and the Declaration of Helsinki. Written informed consent was obtained from patients before study-specific procedures were started.
Eligible patients were 18 to 75 years old who had coronary artery disease and were hospitalized for acute coronary syndrome within 48 hours of the ischemic symptoms. Patients with non–ST-segment elevation acute coronary syndrome and those with ST-segment elevation acute coronary syndrome who received optimal reperfusion therapy (successful treatment with a thrombolytic agent or primary catheter-based intervention initiated within 12 hours of symptom onset) were eligible to enter the study. Patients with non–ST-segment elevation acute coronary syndrome included those with non–ST-segment elevation myocardial infarction and those with unstable angina in whom conservative management was planned. Patients were also required to have an LDL cholesterol level >70 mg/dl and a fasting triglyceride level <500 mg/dl within 72 hours of symptom onset.
Exclusion criteria included treatment for dyslipidemia with prescription medication within the preceding 4 weeks; current treatment with a depot formulation of progesterone or initiation of other hormone therapy within the previous 3 months; Q-wave myocardial infarction, pulmonary edema, moderate or severe congestive heart failure, acute moderate to severe mitral regurgitation (3 to 4+), acute ventricular septal defect, occurrence of ventricular fibrillation, sustained ventricular tachycardia, complete heart block, new-onset atrial fibrillation with an uncontrolled ventricular rate (>100 beats/min), paced ventricular rhythm, stroke, sepsis, acute pericarditis, or any evidence of systemic or pulmonary embolus within the preceding 4 weeks; coronary artery bypass graft within the preceding 3 months; percutaneous coronary intervention within the preceding 6 months to minimize the likelihood that a complication of that intervention would occur during the course of the study; planned therapeutic coronary intervention (other than primary angioplasty) or bypass surgery during current hospitalization; or failed revascularization during current hospitalization. Other exclusion criteria were a history of hypersensitivity reactions to 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, women who were pregnant or breastfeeding, uncontrolled diabetes mellitus, hypertension, hypothyroidism, systolic hypotension, active liver disease or dysfunction, serum creatinine level >2 mg/dl, severe anemia (hematocrit <28%), and serum creatine kinase >3 times the upper limit of normal not caused by myocardial injury.
This trial was a prospective, multicenter, randomized, open-label, 3-arm, parallel-group, phase IIIb study ( http://clinicaltrials.gov , identifier NCT00214630 ) conducted from December 14, 2003, through August 31, 2007, in 169 study centers (166 in the United States, 2 in Costa Rica, 1 in Panama). Consented patients (hospitalized for acute coronary syndrome within 48 hours of initial symptoms) entered a screening period of up to 3 days, during which core laboratory studies were obtained to document the absence of safety issues precluding statin therapy. Eligible patients were randomized in a 1:1:1 ratio to once-daily treatment with RSV20, RSV40, or ATV80 for 12 weeks. Patients were assessed at weeks 2, 6, and 12 after treatment initiation. Average time from symptom onset to first blood analyses was 1.3 days, and average time from symptom onset to randomization to study drug treatment was 3.9 days.
Investigators were blinded to measurements of primary and secondary end-point parameters. The primary end point was efficacy of RSV20 and RSV40 compared with that of ATV80 in lowering LDL cholesterol (direct measurement) averaged over measurements at 6 and 12 weeks. Secondary end points included (1) efficacy of RSV20 and RSV40 versus ATV80 on percent change from baseline in LDL cholesterol at 2, 6, and 12 weeks; (2) percent change from baseline in total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, non-HDL cholesterol, apolipoprotein AI, apolipoprotein B, LDL cholesterol/HDL cholesterol, total cholesterol/HDL cholesterol, non-HDL cholesterol/HDL cholesterol, apolipoprotein B/apolipoprotein AI, and LDL cholesterol (Friedewald calculation ) averaged over 6 to 12 weeks and at 2, 6, and 12 weeks; and (3) percent change from baseline in the inflammatory marker high-sensitivity C-reactive protein averaged over 6 to 12 weeks.
Safety and tolerability were evaluated by recording the incidence and severity of adverse events, abnormal physical examination findings, and abnormal laboratory values through 12 weeks of treatment.
The primary efficacy analysis was based on the intention-to-treat population (patients who had a baseline measurement, had ≥1 measurement after baseline, and had taken ≥1 dose of study medication). Analyses were performed using a last-observation-carried-forward method on the intention-to-treat population for all efficacy variables. Analysis of covariance was used to compare least squares mean differences for the primary end point and percent change from baseline in LDL cholesterol averaged over measurements at 6 and 12 weeks, with a main effect for treatment and baseline LDL cholesterol as a covariate. Sequential noninferiority and superiority tests were used to compare RSV40 with ATV80 and RSV20 with ATV80. Treatment differences were considered statistically significant at a p value <0.05. Secondary end points were analyzed using the same approach, but only superiority tests were performed. Safety data were summarized for the safety population (patients who took ≥1 dose of study medication) using descriptive statistics.
To provide 90% power to detect superiority if the real treatment difference was ≥4%, it was estimated that 621 patients (207 in each treatment arm) would be required in the intention-to-treat population.
Results
In total 1,391 patients entered the screening period, and 566 failed screening. The most common reason for screen failure (81%) was not meeting the entry criteria. In total 825 patients were randomized to treatment, and 799 of these received ≥1 dose of study medication and were evaluated for safety. The intention-to-treat population comprised 754 patients.
Demographic and clinical characteristics of the 825 randomized patients were well balanced among the 3 treatment arms ( Table 1 ). Overall, most patients were men (76%), white (80%), and <65 years old (89%). Obesity (body mass index >30 kg/m 2 ) was common (335 of 825, 41%). Myocardial infarction was the most common reason for hospital admission, with similar percentages of patients having ST-segment elevation myocardial infarction (320 of 825, 39%) and non–ST-segment elevation myocardial infarction (294 of 825, 36%); unstable angina was a less common reason for admission (211 of 825, 26%). As entry criteria, all patients with ST-segment elevation myocardial infarction were required to have had successful treatment with a thrombolytic agent or primary catheter-based intervention within 12 hours of symptom onset.
| Variable | RSV20 | RSV40 | ATV80 |
|---|---|---|---|
| (n = 277) | (n = 270) | (n = 278) | |
| Gender | |||
| Men | 207 (74.7%) | 200 (74.1%) | 219 (78.8%) |
| Women | 70 (25.3%) | 70 (25.9%) | 59 (21.2%) |
| Age (years) | |||
| Mean ± SD | 53.0 ± 9.0 | 52.8 ± 8.8 | 52.9 ± 9.4 |
| Range | 28–73 | 23–72 | 19–75 |
| Race or ethnicity | |||
| White | 216 (78.0%) | 226 (83.7%) | 221 (79.5%) |
| Black | 34 (12.3%) | 26 (9.6%) | 34 (12.2%) |
| Hispanic | 18 (6.5%) | 10 (3.7%) | 14 (5.0%) |
| Asian | 1 (0.4%) | 2 (0.7%) | 3 (1.1%) |
| Other | 8 (2.9%) | 6 (2.2%) | 6 (2.2%) |
| Type of acute coronary syndrome | |||
| ST-segment elevation myocardial infarction | 113 (40.8%) | 100 (37.0%) | 107 (38.5%) |
| Non–ST-segment elevation myocardial infarction | 89 (32.1%) | 101 (37.4%) | 104 (37.4%) |
| Unstable angina | 75 (27.1%) | 69 (25.6%) | 67 (24.1%) |
| Body mass index (kg/m 2 ) | (n = 266) | (n = 264) | (n = 269) |
| Mean ± SD | 29.4 ± 5.3 | 30.4 ± 6.0 | 30.4 ± 5.9 |
| >30 | 106 (38.3%) | 118 (43.7%) | 111 (39.9%) |
| Medical history | |||
| Myocardial infarction/acute coronary syndrome | 30 (10.8%) | 39 (14.4%) | 29 (10.4%) |
| Coronary artery disease | 46 (16.6%) | 55 (20.4%) | 37 (13.3%) |
| Percutaneous coronary intervention | 65 (23.5%) | 55 (20.4%) | 50 (18.0%) |
| Coronary bypass | 5 (1.8%) | 6 (2.2%) | 9 (3.2%) |
| Hypertension | 144 (52.0%) | 137 (50.7%) | 139 (50.0%) |
| Diabetes | 32 (11.6%) | 35 (13.0%) | 36 (16.5%) |
| Hyperlipidemia ⁎ | 83 (30.0%) | 83 (30.7%) | 65 (23.4%) |
| Smoker | 40 (14.4%) | 44 (16.3%) | 50 (18.0%) |
⁎ Reported by investigators as history of dyslipidemia, hyperlipidemia, or increased cholesterol.
At baseline mean LDL cholesterol was similar in the 3 treatment arms and within the range of 133 to 139 mg/dl ( Table 2 ). Mean change from baseline in LDL cholesterol averaged over weeks 6 and 12 was significantly greater with RSV40 compared with ATV80 (p = 0.02; Table 2 ). LDL cholesterol lowering by RSV20 was similar to that by ATV80. Similar results were achieved in all subcategories of acute coronary syndrome (unstable angina, non–ST-segment elevation myocardial infarction, and ST-segment elevation myocardial infarction). There was also no difference observed in obese versus nonobese patients. Sensitivity analysis of LDL cholesterol calculated using the Friedewald equation yielded results that concurred with those from the primary analysis of LDL cholesterol by direct measurement ( Supplemental Table 1 ).
| Variable | RSV20 | RSV40 | ATV80 |
|---|---|---|---|
| (n = 246) | (n = 251) | (n = 257) | |
| Low-density lipoprotein cholesterol (mg/dl) | |||
| Mean baseline | 138.4 | 138.8 | 133.2 |
| Percent change, mean ± SD | −42.0 ± 18.5 | −46.8 ⁎ ± 18.2 | −42.7 ± 17.7 |
| High-density lipoprotein cholesterol (mg/dl) | |||
| Mean baseline | 39.5 | 38.8 | 39.9 |
| Percent change, mean ± SD | 9.7 † ± 16.4 | 11.9 ‡ ± 19.7 | 5.6 ± 19.1 |
| Non–high-density lipoprotein cholesterol (mg/dl) | |||
| Mean baseline | 161.2 | 162.8 | 156.0 |
| Percent change, mean ± SD | −37.9 ± 73.3 | −42.6 ± 17.6 | −39.8 ± 17.4 |
| Total cholesterol (mg/dl) | |||
| Mean baseline | 200.7 | 201.7 | 195.9 |
| Percent change, mean ± SD | −28.6 ⁎ ± 15.4 | −32.2 ± 15.7 | −30.9 ± 15.1 |
| Triglycerides (mg/dl) | |||
| Subjects | 254 | ||
| Mean baseline | 180.8 | 182.7 | 157.5 |
| Percent change, mean ± SD | −9.5 † ± 40.4 | −14.6 ± 48.3 | −18.0 ± 38.7 |
| Low-density lipoprotein cholesterol/high-density lipoprotein cholesterol | |||
| Mean baseline | 3.68 | 3.77 | 3.59 |
| Percent change, mean ± SD | −46.5 ± 16.5 | −51.5 ‡ ± 16.6 | −44.5 ± 18.0 |
| Non–high-density lipoprotein cholesterol/high-density lipoprotein cholesterol | |||
| Mean baseline | 4.32 | 4.46 | 4.25 |
| Percent change, mean ± SD | −42.3 ± 16.8 | −47.3 ‡ ± 17.3 | −41.2 ± 19.0 |
| Total cholesterol/high-density lipoprotein cholesterol | |||
| Mean baseline | 5.32 | 5.46 | 5.25 |
| Percent change, mean ± SD | −34.0 ± 14.1 | −38.2 ‡ ± 14.5 | −33.1 ± 15.6 |
| Apolipoprotein B (mg/dl) | |||
| Subjects | 223 | 224 | 231 |
| Mean baseline | 130.0 | 132.2 | 127.4 |
| Percent change, mean ± SD | −34.2 ± 15.9 | −37.9 ± 15.0 | −36.3 ± 17.1 |
| Apolipoprotein AI (mg/dl) | |||
| Subjects | 223 | 224 | 231 |
| Mean baseline | 134.6 | 134.0 | 135.3 |
| Percent change, mean ± SD | 10.3 † ± 25.3 | 10.1 ‡ ± 14.8 | 4.2 ± 16.0 |
| Apolipoprotein B/apolipoprotein AI | |||
| Subjects | 223 | 224 | 231 |
| Mean baseline | 1.00 | 1.01 | 0.97 |
| Percent change, mean ± SD | −39.4 ± 14.1 | −43.0 † ± 14.6 | −38.3 ± 15.4 |
| High-sensitivity C-reactive protein | |||
| Subjects | 238 | 241 | 249 |
| Median baseline (mg/L) | 12.30 | 12.90 | 12.30 |
| Percent change, mean ± SD | −84.9 ± 768.3 | −83.0 ± 53.1 | −85.0 ± 42.6 |
LDL cholesterol had decreased to approximately its final values in all 3 groups by 2 weeks after starting treatment; subsequent changes from week 2 to weeks 6 and 12 were of smaller magnitude ( Figure 1 ) . Decrease in LDL cholesterol with RSV40 was significantly greater than that with ATV80 at week 12 (p = 0.02) but not at weeks 2 and 6. Decrease in LDL cholesterol with RSV20 was similar to that with ATV80 at week 12 but was significantly less at weeks 2 (p <0.01) and 6 (p = 0.04).
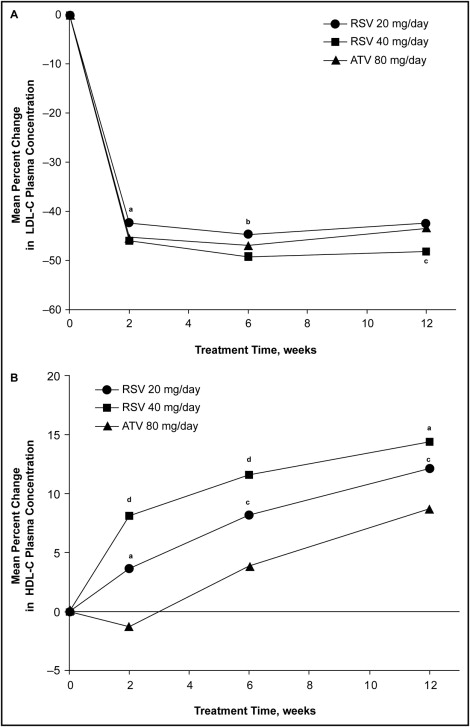
Mean baseline HDL cholesterol was similar across the 3 treatment arms (∼39 mg/dl; Table 2 ). Mean change from baseline in HDL cholesterol averaged over weeks 6 and 12 showed that HDL cholesterol increased by a significantly greater extent with RSV20 (p <0.01) and RSV40 (p <0.001) than with ATV80 ( Table 2 ).
At week 2, HDL cholesterol increased by 3.6% with RSV20 and 8.1% with RSV40 but decreased by 1.3% with ATV80 ( Figure 1 ). At weeks 6 and 12, HDL cholesterol increased with all 3 treatments. Compared with ATV80, increases in HDL cholesterol were significantly greater with RSV20 (p <0.05) and RSV40 (p <0.01) at weeks 2, 6, and 12.
RSV40 was significantly more effective than ATV80 in improving apolipoprotein AI (p <0.001) and several lipid ratios, including LDL cholesterol/HDL cholesterol (p <0.001), non-HDL cholesterol/HDL cholesterol (p <0.001), total cholesterol/HDL cholesterol (p <0.001), and apolipoprotein B/apolipoprotein AI (p <0.01; Table 2 ). RSV20 was significantly more effective than ATV80 in increasing apolipoprotein AI (p <0.01) but was significantly less effective in decreasing total cholesterol (p <0.05) and triglycerides (p <0.01; Table 2 ). Changes in non-HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein with RSV20 and RSV40 were not significantly different from those with ATV80 ( Table 2 ).
Serious adverse events occurred in 14.1% of patients treated with ATV80, 10.5% of those treated with RSV20, and 8.7% of those treated with RSV40 ( Table 3 ). Serious cardiovascular adverse events were infrequently observed in any treatment group ( Table 3 ). None of the serious adverse events or serious cardiovascular adverse events was considered by the investigators to be related to study treatment. Discontinuation of study treatment because of an adverse event occurred in 3.7%, 6.1%, and 9.3% of patients treated with RSV20, RSV40, and ATV80, respectively ( Table 3 ). Musculoskeletal and connective tissue abnormalities accounted for most study discontinuations because of an adverse event. Three patients died during the study ( Table 3 ): a 66-year-old man treated with RSV40 died of myocardial infarction on treatment day 1, a 51-year-old man treated with RSV40 died of cardiac arrest secondary to ventricular fibrillation on treatment day 3, and a 41-year-old man treated with ATV80 died of torsade de pointes on treatment day 2. None of these deaths was judged by the investigators to be related to study treatment.



