Statin therapy produces regression of coronary artery plaques and reduces the incidence of coronary artery disease. However, not all patients show regression of coronary atherosclerosis after statin therapy. The purpose of the present study was to identify differences in clinical characteristics, serum lipid profiles, arterial remodeling, and plaque composition between patients with progression and those with regression of coronary atherosclerosis during statin therapy. The effects of 8-month statin therapy on coronary atherosclerosis were evaluated in the Treatment With Statin on Atheroma Regression Evaluated by Intravascular Ultrasound With Virtual Histology (TRUTH) study using intravascular ultrasound–virtual histology. One hundred nineteen patients were divided into 2 groups according to atheroma volume increase (progressors) or decrease (regressors) during an 8-month follow-up period. Fifty-one patients (43%) were categorized as progressors and the remaining 68 (57%) as regressors. External elastic membrane volume increased, although not significantly (0.8%, p = 0.34), and luminal volume decreased significantly (−5.3%, p = 0.0003) in progressors, while external elastic membrane volume decreased significantly (−3.2%, p <0.0001) and luminal volume increased (2.2%, p = 0.13) in regressors. The fibrous component increased significantly in progressors, while this component decreased in regressors. A strong positive correlation was observed between change in atheroma volume and change in fibrous volume (r = 0.812, p <0.0001). In conclusion, coronary arteries showed negative remodeling during statin-induced plaque regression. The difference in plaque composition between patients with progression and those with regression of coronary atherosclerosis during statin therapy arose from the difference in the change in fibrous component.
Recent trials using grayscale intravascular ultrasound (IVUS) have shown that intensive lipid-lowering therapy with statins results in plaque regression. However, not all patients show regression of coronary atherosclerosis under statin therapy. Some patients show plaque progression and develop coronary events under statin therapy. The evaluation of tissue characteristics is important for understanding the process of plaque progression or regression. However, conventional grayscale IVUS is significantly limited for accurately assessing plaque composition. Therefore, in the present study, we performed additional analysis of the Treatment With Statin on Atheroma Regression Evaluated by Intravascular Ultrasound With Virtual Histology (TRUTH) study to evaluate whether there were differences in clinical characteristics, serum lipid profiles, arterial remodeling, and plaque composition between patients with progression and those with regression of coronary atherosclerosis during statin therapy.
Methods
The present study is a subanalysis of the TRUTH study. The TRUTH study was a prospective, open-labeled, randomized, multicenter trial performed at 11 Japanese centers to evaluate the effects of 8-month treatment with pitavastatin versus pravastatin on coronary artery plaque composition using IVUS–virtual histology (VH). Details of the study design have been reported previously. Briefly, 164 patients with angina pectoris were randomized to either pitavastatin (4 mg/day; intensive lipid lowering) or pravastatin (20 mg/day; moderate lipid lowering) therapy after successful percutaneous coronary intervention under IVUS-VH guidance. Follow-up IVUS examinations were performed after 8 months of statin therapy. We used the full analysis set of the TRUTH study for this subanalysis. Patients were included in the full analysis set if they had measurable IVUS lesions at enrollment and at follow-up. A total of 119 patients in the full analysis set were divided into 2 groups: progressors and regressors. A progressor was defined as plaque-plus-media volume at follow-up – plaque-plus-media volume at baseline ≥ 0. A regressor was defined as plaque-plus-media volume at follow-up – plaque-plus-media volume at baseline < 0.
There are 2 distinct classes of statins: lipophilic (pitavastatin, atorvastatin, fluvastatin, and simvastatin) and hydrophilic (pravastatin and rosuvastatin). Pitavastatin is a commonly used statin in Japan, and its ability to lower serum low-density lipoprotein (LDL) cholesterol is comparable to that of atorvastatin. The most effective pitavastatin dose for reducing serum LDL cholesterol has been shown to be 4 mg/day in Japan. This dose has been shown to be effective for lowering serum LDL cholesterol to the same level as that of atorvastatin 20 mg/day.
The TRUTH study was conducted in accordance with the Declaration of Helsinki and with the approval of the institutional ethics committees of the 11 participating institutions. Written informed consent was obtained from each patient enrolled in the study.
Details of the IVUS procedure and examination are documented elsewhere. Briefly, after percutaneous coronary intervention of the culprit lesion, IVUS examination was performed for angiographic lesions with <50% luminal narrowing on the distal and proximal sides of the culprit lesion. An IVUS catheter (Eagle Eye Gold; Volcano Corporation, San Diego, California) was used, and a motorized pullback device withdrew the transducer at 0.5 mm/s. During pullback, grayscale IVUS was recorded and raw radiofrequency data were captured at the top of the R wave using a commercially available IVUS console (IVG3; Volcano Corporation). After 8 months of statin therapy, IVUS examination was repeated in the same coronary artery using the same type of IVUS catheter as at baseline.
All baseline and follow-up IVUS core laboratory analyses were performed by an independent and experienced investigator (M.T.) in a blinded manner. Before IVUS analysis, baseline and follow-up IVUS images were reviewed side by side on a display, and the distal and proximal ends of the target segment were identified on the basis of the presence of reproducible anatomic landmarks such as the side branch, vein, and stent edge. The target segment of interest had ≥50% plaque burden according to IVUS. Plaques close to the percutaneous coronary intervention site (those within 5 mm) were excluded. In patients who underwent multivessel percutaneous coronary intervention, the vessel with the greatest atheroma volume was selected. Manual contour detection of the lumen and the external elastic membrane (EEM) was performed for each frame. Quantitative IVUS grayscale analysis was performed according to the guidelines of the American College of Cardiology and the European Society of Cardiology. All volumetric data were divided by lesion length to obtain a volume index. IVUS-VH data analysis was based on grayscale border contour calculation, and relative and absolute amounts of different coronary artery plaque components were measured using IVUSLab version 2.2 (Volcano Corporation).
Blood examinations for lipid levels were performed at baseline and at 8-month follow-up. The levels of serum lipids, apolipoproteins, and high-sensitivity C-reactive protein were measured at a central clinical laboratory (SRL, Inc., Tokyo, Japan).
The necessary sample size was estimated on the basis of the assumption that changes in plaque composition are accompanied by changes in atheroma volume. The percentage change in atheroma volume was estimated on the basis of a previous report, and it was 11.5% in the pitavastatin group and 5.3% in the pravastatin group. The standard deviation of atheroma volume percentage changes after statin therapy was 12.8%. On the basis of these factors, the required sample size in this study was 69 patients in each group to achieve 80% power using a 2-sided, 2-sample Student’s t test at a significance level of 5%. Assuming that 10% of patients would drop out of the study, the sample size per group was determined to be 77 patients.
Statistical analyses were performed using StatView version 5.0 (SAS Institute Inc., Cary, North Carolina). Results are expressed as mean ± SD or as median (range). Differences in continuous variables between the 2 groups were compared using unpaired Student’s t tests when the variables showed a normal distribution and using Mann-Whitney U tests when they did not. Differences in continuous variables within each group were compared using paired Student’s t tests when the variables showed a normal distribution and using Wilcoxon’s signed rank-sum tests when they did not. Categorical variables were compared between the 2 groups using chi-square tests or Fisher’s exact tests. Univariate linear regression analyses were performed to assess the relations between change in atheroma volume and 4 plaque components. Statistical significance was set at p <0.05.
Results
Baseline characteristics of the subjects are listed in Table 1 . Fifty-one patients (43%) were included in the progressor group, and the remaining 68 patients (57%) were in the regressor group. The following characteristics were significantly higher in progressors: mean age, frequency of hypertension, and use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. The frequency of diabetes in progressors was also higher than that in regressors, although this difference was not statistically significant.
| Variable | Progressors | Regressors | p Value |
|---|---|---|---|
| (n = 51) | (n = 68) | ||
| Age (years) | 70 ± 9 | 64 ± 10 | 0.002 |
| Men | 41 (80%) | 58 (85%) | 0.21 |
| Body mass index (kg/m 2 ) | 24.2 ± 2.5 | 24.7 ± 3.9 | 0.45 |
| Diabetes mellitus | 26 (51%) | 24 (35%) | 0.09 |
| Hypertension | 39 (76%) | 36 (53%) | 0.009 |
| Treatment allocation | 0.29 | ||
| Pitavastatin | 22 (43%) | 36 (53%) | |
| Pravastatin | 29 (57%) | 32 (47%) | |
| Status of coronary artery disease | 0.56 | ||
| Stable angina pectoris | 37 (73%) | 46 (68%) | |
| Unstable angina pectoris ⁎ | 14 (27%) | 22 (32%) | |
| Number of disease vessels | 0.72 | ||
| 1 | 22 (43%) | 37 (54%) | |
| 2 | 22 (43%) | 23 (34%) | |
| 3 | 7 (14%) | 8 (12%) | |
| Target coronary artery | 0.95 | ||
| Left anterior descending | 28 (55%) | 39 (57%) | |
| Left circumflex | 2 (4%) | 3 (4%) | |
| Right | 21 (41%) | 26 (38%) | |
| Multivessel percutaneous coronary intervention | 5 (10%) | 3 (4%) | 0.43 |
| Type of stent | 0.48 | ||
| Bare metal stent | 10 (20%) | 10 (15%) | |
| Drug-eluting stent | 41 (80%) | 58 (85%) | |
| Number of stents | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.26 |
| Antiplatelet therapy | |||
| Aspirin | 51 (100%) | 66 (97%) | 0.51 |
| Thienopyridines | 51 (100%) | 67 (99%) | >0.99 |
| Other medications | |||
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 32 (63%) | 29 (43%) | 0.03 |
| β blockers | 6 (12%) | 7 (10%) | 0.8 |
| Calcium channel blockers | 30 (59%) | 30 (44%) | 0.11 |
| Heart rate (beats/min) | 70 ± 12 | 71 ± 12 | 0.48 |
| Follow-up duration (days) | 233 ± 38 | 222 ± 35 | 0.11 |
Risk factor control at baseline and at follow-up is listed in Table 2 . Serum LDL cholesterol level decreased significantly from 125 to 79 mg/dl in progressors and from 137 to 89 mg/dl in regressors; LDL cholesterol level at follow-up was significantly lower in progressors than in regressors. A significant increase in high-density lipoprotein cholesterol level and a decrease in oxidized LDL level were observed only in regressors. The median high-sensitivity C-reactive protein level decreased significantly in the 2 groups.
| Variable | Progressors (n = 51) | Regressors (n = 68) | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p Value | Baseline | Follow-Up | p Value | |
| Total cholesterol (mg/dl) | 198 ± 37 | 152 ± 28 ⁎ | <0.0001 | 210 ± 34 | 164 ± 28 | <0.0001 |
| % change | −22 ± 13 | −21 ± 12 | ||||
| LDL cholesterol (mg/dl) | 125 ± 33 | 79 ± 24 ⁎ | <0.0001 | 137 ± 31 | 89 ± 25 | <0.0001 |
| % change | −36 ± 17 | −34 ± 15 | ||||
| Triglycerides (mg/dl) | 121 ± 49 | 109 ± 55 | 0.08 | 139 ± 75 | 123 ± 69 | 0.1 |
| % change | −6 ± 37 | −4 ± 47 | ||||
| High-density lipoprotein cholesterol (mg/dl) | 48 ± 12 | 50 ± 18 | 0.11 | 45 ± 11 | 51 ± 13 | 0.0001 |
| % change | 7 ± 22 | 15 ± 25 | ||||
| Apolipoprotein A-I (mg/dl) | 119 ± 21 | 130 ± 22 | 0.0007 | 116 ± 19 | 134 ± 27 | <0.0001 |
| % change | 10 ± 18 | 15 ± 18 | ||||
| Apolipoprotein B (mg/dl) | 97 ± 23 † | 68 ± 17 † | <0.0001 | 109 ± 22 | 78 ± 17 | <0.0001 |
| % change | −29 ± 16 | −27 ± 13 | ||||
| High-sensitivity C-reactive protein (ng/ml) | 2790 (193 to 62900) | 729 (52 to 23200) | 0.003 | 5120 (54 to 88900) | 628 (109 to 26200) | 0.0001 |
| % change | −74 (−100 to 636) | −77 (−100 to 275) | ||||
| Oxidized LDL (U/ml) | 11.2 ± 8.0 | 12.8 ± 15.2 | 0.61 | 11.6 ± 8.3 | 9.7 ± 6.4 | 0.004 |
| % change | 29 ± 171 | −10 ± 31 | ||||
| Glucose (mg/dl) | 116 ± 42 | 110 ± 32 | 0.12 | 112 ± 29 | 107 ± 33 | 0.05 |
| % change | −4 ± 25 | −4 ± 21 | ||||
| Systolic blood pressure (mm Hg) | 138 ± 22 | 135 ± 22 | 0.33 | 131 ± 22 | 133 ± 23 | 0.45 |
| % change | −1 ± 16 | 3± 17 | ||||
| Diastolic blood pressure (mm Hg) | 77 ± 15 | 75 ± 14 | 0.48 | 75 ± 11 | 76 ± 11 | 0.65 |
| % change | 0 ± 18 | 3 ± 19 | ||||
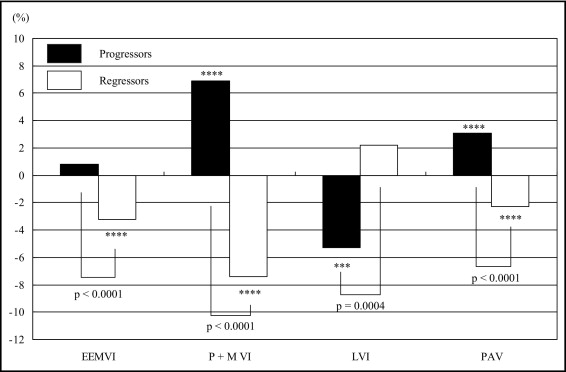
Parameters evaluated using grayscale IVUS are listed in Table 3 . Percentage atheroma volume increased significantly in progressors, while this value decreased in regressors. EEM volume increased, although not significantly, from 16.36 to 16.48 mm 3 /mm, and luminal volume decreased significantly from 7.60 to 7.17 mm 3 /mm in progressors. In contrast, EEM volume decreased significantly from 16.34 to 15.81 mm 3 /mm and luminal volume increased from 7.26 to 7.41 mm 3 /mm in regressors. There were significant differences in the mean change in percentage atheroma volume and the mean percentage changes of EEM volume, atheroma volume, and luminal volume between the 2 groups ( Figure 1 ) .
| Variable | Progressors (n = 51) | Regressors (n = 68) | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p Value | Baseline | Follow-Up | p Value | |
| EEM volume index | ||||||
| Mean (mm 3 /mm) | 16.36 ± 5.53 | 16.48 ± 5.64 | 0.34 | 16.34 ± 5.17 | 15.81 ± 5.07 | <0.0001 |
| Mean % change | 0.8 ± 4.9 ‡ | −3.2 ± 5.6 | ||||
| Plaque-plus-media volume index | ||||||
| Mean (mm 3 /mm) | 8.76 ± 3.39 | 9.31 ± 3.47 | <0.0001 | 9.08 ± 3.26 | 8.40 ± 2.97 | <0.0001 |
| Mean % change | 6.9 ± 5.8 ‡ | −7.4 ± 5.7 | ||||
| Luminal volume index | ||||||
| Mean (mm 3 /mm) | 7.60 ± 2.96 | 7.17 ± 2.83 | 0.0003 | 7.26 ± 2.31 | 7.41 ± 2.53 | 0.13 |
| Mean % change | −5.3 ± 10.6 † | 2.2 ± 11.5 | ||||
| Percentage atheroma volume | ||||||
| Mean (%) | 53.5 ± 8.2 | 56.6 ± 7.4 ⁎ | <0.0001 | 55.4 ± 5.9 | 53.0 ± 6.3 | <0.0001 |
| Mean nominal change (%) | 3.1 ± 3.2 ‡ | −2.3 ± 3.1 | ||||
| Average length (mm) | 24.3 ± 14.9 | — | 24.8 ± 15.0 | — | ||
Parameters evaluated using IVUS-VH are listed in Table 4 . A significant decrease in the fibrofatty component and an increase in the dense calcium component were observed in the 2 groups. The necrotic core component increased significantly in progressors, and it tended to increase in regressors. The fibrous component increased significantly in progressors, while this value decreased in regressors. Significant differences were observed in the fibrous component volume change ( Figure 2 ) and the fibrous volume at follow-up between the 2 groups ( Table 4 ). A strong positive correlation between changes in atheroma volume and fibrous volume ( r = 0.812, p <0.0001) and a weak positive correlation between changes in atheroma volume and fibrofatty volume ( r = 0.331, p = 0.002) were observed, while there were no correlations between changes in atheroma volume and dense calcium volume or necrotic core volume ( Figure 3 ) .



