Chapter 67 Clinical Use and Mechanistic Implications of Microvolt T-Wave Alternans and Signal-Averaged Electrocardiography
Microvolt T-Wave Alternans
Elizabeth S. Kaufman, Michael J. Cutler, David S. Rosenbaum
Introduction
The significance of repolarization alternans, a beat-to-beat alternation in T-wave morphology, was described almost a century ago by Sir Thomas Lewis, who noted that “alternation occurs either when the heart muscle is normal but the heart rate is very fast or when there is serious heart disease and the rate is normal.”1 In the past 25 years, clinicians have learned to exploit the phenomenon of T-wave alternans to identify serious heart disease, especially to detect patients who are at high risk of ventricular tachyarrhythmias and SCD. Whereas earlier investigators observed visible T-wave alternans in patients at risk of imminent SCD, including patients with Prinzmetal’s angina, electrolyte disorder, or QT prolongation, more recent investigators have used techniques capable of detecting invisible MTWA during modestly increased heart rate to identify patients with a significantly increased long-term risk of SCD.2–4 In the recent past, there has also arisen a need to identify patients with advanced structural heart disease whose risk of SCD is low. That is, in an age when the ICD is widely accepted as an effective (albeit expensive) means of preventing SCD, the perception of a need to identify low-risk patients who may not require an ICD is also growing.5,6 There are two major reasons for this. First, a strategy that can divert low-risk patients away from ICD implantation will increase the cost-effectiveness of ICD therapy. Second, the ability to identify low-risk patients could lead to an increase in the appropriate use of ICD therapy. There is evidence that patients who meet current criteria for primary prevention ICD implantation (based on ejection fraction alone) often do not receive ICDs.7 It is reasonable to think that both physicians and patients are more likely to accept ICD therapy if the need for such therapy can be more clearly defined. MTWA testing has shown promise for detecting patients at low risk for SCD.
In parallel with advances in the understanding of the clinical implications of MTWA testing, new insights into the cellular and tissue-level mechanisms of T-wave alternans have also become available. Experimental models have shown that T-wave alternans occurs during alternation of action potential morphology, which may occur heterogeneously throughout the heart and lead to electrical instability.8 Evidence suggests that alternans of calcium cycling within the sarcoplasmic reticulum may underlie T-wave alternans.9
Mechanisms of T-Wave Alternans
In 1999, Pastore et al used high-resolution optical mapping with voltage-sensitive dye in a guinea pig model to measure alternans of action potential duration in different areas of the myocardium.8 Above a critical heart rate, groups of cells alternated out of phase with other nearby cells (i.e., short-long-short action potentials in one area coincided with long-short-long action potentials nearby). This situation led to re-entry and VF, demonstrating the mechanism linking alternans with ventricular tachyarrhythmias and SCD.
Two leading hypotheses for the underlying electrophysiological mechanism of action potential duration alternans have been proposed. (1) The restitution hypothesis states that alternans occurs when the cell membrane’s ion channels are not able to produce complete repolarization in time for the next action potential. Although this may be one mechanism involved in alternans, it fails to explain some observations (e.g., why alternans is seen in ischemia when the restitution slope is flat).10 (2) The other hypothesis is that alternans occurs when the heart rate exceeds the capability of cellular calcium cycling mechanisms to maintain intracellular calcium homeostasis. Pruvot et al measured simultaneous action potentials and calcium (Ca2+) transients in the guinea pig model and found consistent parallels between action potential and Ca2+ alternans both spatially and temporally. They concluded that T-wave alternans is closely associated with Ca2+ cycling. To further support the Ca2+ cycling hypothesis, reduced expression of SR Ca2+-adenosine triphosphatase, which reclaims cytosolic Ca2+, or impaired ryanodine receptor function, which is responsible for the release of Ca2+ from the sarcoplasmic reticulum, are associated with Ca2+ alternans and action potential duration alternans.11,12
Measurement of Microvolt T-Wave Alternans in Clinical Practice
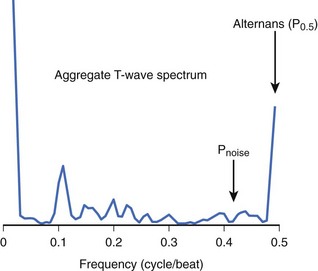
Although MTWA is relatively common in high-risk patients, there are several obstacles to detecting such small (microvolt-level) fluctuations in the T wave and, in particular, to distinguishing MTWA from other sources of fluctuation in the electrocardiogram (ECG) signal. Sources of confounding signal variation include variation in heart rhythm (such as sinus arrhythmia or atrial or ventricular ectopy), respiration (a periodic source of noise), and noise at the patient-electrode interface. To detect MTWA and distinguish it from other signal variations, the spectral method was developed.13 This method involves measuring the amplitude of the T wave in sequential beats to create a time series, which is then analyzed using fast Fourier transform to produce a power spectrum (Figure 67-1). The power at 0.5 cycles/beat corresponds to every-other-beat MTWA. MTWA is reported in microvolts as alternans voltage, the square root of the power. Along with alternans voltage, alternans ratio (k) is also reported. This is alternans power divided by the standard deviation of the background noise. In contrast, a nonspectral method, called modified moving average analysis, divides beats into even and odd bins and averages the morphology of T waves in each bin over several beats at a time.14 The difference in amplitude of the odd beats versus the even beats is a measure of alternans. The modified moving average technique is susceptible to false detection of alternans in the presence of noise.15 Moreover, this technique has not yet been validated adequately as a long-term clinical risk predictor.
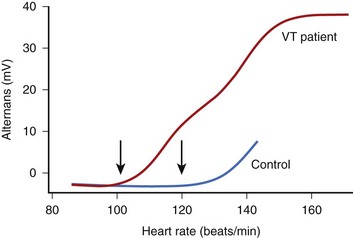
The onset of MTWA occurs when an individual’s heart rate exceeds a particular threshold heart rate, and MTWA tends to persist while the heart rate remains higher than that threshold heart rate.13,16 The threshold heart rate is specific to an individual. Whereas in normal subjects, MTWA may appear at high heart rates, in patients with structural heart disease and high risk of death or sustained ventricular arrhythmias, the threshold heart rate for MTWA tends to be less than 110 beats/min (Figure 67-2).17–19 The higher the heart rate at which MTWA is measured, the higher the sensitivity and the lower the specificity for detecting individuals at high risk of ventricular arrhythmia.18 By convention, MTWA that occurs at heart rate of 110 beats/min or less has been defined as abnormal.20 To detect abnormal MTWA, it is therefore necessary to increase the heart rate into the range of 105 to 110 beats/min.
Initial studies of MTWA used atrial pacing to elevate heart rate.21 Subsequently, exercise testing was adopted, and most large clinical trials assessing the value of MTWA testing have been done with exercise. Because the magnitude of MTWA oscillates over time, even at a constant heart rate, in an MTWA study it is important to maintain a subject’s heart rate in the target zone between 100 and 110 beats/min for several minutes.17 Most standard work-based exercise protocols such as the Bruce protocol elevate heart rate too quickly through this critical zone. The performance of a diagnostic MTWA test requires a heart rate–guided exercise protocol tailored to an individual’s level of fitness and heart rate response.
Traditionally, MTWA tests have been classified as positive, negative, or indeterminate.20 A MTWA test is considered “positive” when MTWA appears at an onset heart rate of 110 beats/min or less, with a magnitude of more than 1.9 µV and an alternans ratio greater than 3, and is sustained for at least 1 minute while the heart rate remains over the heart rate threshold. MTWA study results are negative if there is no sustained MTWA at an onset heart rate of 110 beats/min or less and there is at least 1 minute of recording of sinus tachycardia at a heart rate 105 beats/min or greater, during which time there is a low noise level (<2 µV), fewer than 10% ectopic beats, and no nonsustained MTWA. Study results that do not meet either of these criteria are considered indeterminate.
Several factors may interfere with the measurement of MTWA and result in indeterminate study results. Two of these are technical issues: (1) high levels of noise (which can be reduced by careful skin preparation and use of high-resolution electrodes) and (2) rapid elevation of heart rate through the target heart rate zone (which can be avoided by a modified exercise protocol tailored to heart rate). In addition, several patient-related factors interfere with the measurement of MTWA. Failure to elevate heart rate to at least 105 beats/min, dense (usually ventricular) ectopy that persists during exercise, and the occurrence of nonsustained MTWA can also lead to what has been termed an indeterminate test result. Recently, a detailed analysis22 of the causes and outcomes of indeterminate MTWA test results showed that only about 6% of “indeterminate” tests were so classified because of technical factors (noise or rapid heart rate rise); the other 94% were caused by patient factors. The prognostic significance of an indeterminate test was as bad as a positive test result, lending support to the practice of combining positive and indeterminate results into a single abnormal category for the purpose of risk stratification.
There has been debate among MTWA investigators regarding whether β-blocker therapy should be held before testing. When pacing is used to elevate heart rate, β-blockade decreases alternans voltage and converts a substantial number of positive tests to negative.23 β-Blocker therapy may also interfere with achieving the target heart rate of 105 beats/min during an exercise study. However, continuing β-blocker therapy is convenient, and testing on β-blocker may more accurately assess risk in patients who will continue long-term therapy with these medications.24 In the large study by Bloomfield et al, of which the analysis of indeterminate results was a substudy, more than 80% of patients were taking β-blocker medications, which were not held before MTWA testing.22,25 Nevertheless, MTWA testing had excellent predictive accuracy in that study. A meta-analysis by Chan et al showed a much higher predictive power of MTWA in studies that did not withhold β-blockers than in those that did.26
Measurement of MTWA is reproducible when testing is repeated within a short time span.27,28 Few data regarding the long-term stability of MTWA are available. The large studies that have led to the acceptance of MTWA as a prognostic indicator have required sinus rhythm and have been performed with exercise. Preliminary studies suggest that AV sequential pacing and pharmacologic elevation of heart rate may provide additional methods for assessing MTWA, although the prognostic value of such testing has not been validated.29,30 A study by Kraaier et al showed low concordance among exercise, atrial pacing, and AV sequential pacing; the predictive value of these methods has not been established.31
Development of Microvolt T-Wave Alternans as a Tool for Clinical Risk Stratification
Studies Establishing Microvolt T-Wave Alternans as a Marker of Susceptibility to Sudden Cardiac Death
After initial studies in animals showed a relationship between MTWA and vulnerability to ventricular fibrillation, Rosenbaum et al published the first prospective study of MTWA as a predictor of clinical risk.13,21 In this study, atrial pacing was used to elevate heart rate in patients undergoing electrophysiological studies. The presence of MTWA at a paced heart rate of 100 beats/min was related to inducibility of ventricular tachycardia (VT) and to the risk of future arrhythmic events. Subsequently, Hohnloser and Estes demonstrated that noninvasive exercise testing could yield MTWA results that were comparable with those seen with atrial pacing.16,32 Additional studies showed that MTWA assessed during exercise performed well as a predictor of future ventricular arrhythmic events compared with other noninvasive risk stratifiers, even with invasive electrophysiological studies.33–36 A consistent finding in these studies was the strong negative predictive value of a negative MTWA test.
However, not all studies showed a high positive predictive value of the MTWA test. Tapanainen et al assessed MTWA in patients with acute myocardial infarction during the predischarge exercise test (when many patients could not or were not pushed to achieve an adequate heart rate) and found that an indeterminate or incomplete MTWA study was strongly associated with risk of death; the negative predictive accuracy of an MTWA study was 99%.37 In the Marburg Cardiomyopathy Study, a positive MTWA was not a significant risk predictor, although an indeterminate test was associated with significant ventricular arrhythmia.38 Interestingly, the patients in the Marburg study had MTWA analysis performed off β-blocker therapy. It may be that MTWA testing performed on β-blocker therapy correlates better with chronic risk for patients on this background therapy, as suggested by Klingenheben et al.24 As noted above, a recent meta-analysis by Chan et al showed a much higher predictive power of MTWA in studies that did not withhold β-blockers than in those that did.26
Microvolt T-Wave Alternans in Patients with Depressed Ejection Fraction After Myocardial Infarction
The results of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) showed a reduction in the mortality rate of patients with chronic ischemic heart disease and ejection fraction 0.30 or less with prophylactic ICD implantation, but at a great cost (approximately 17 ICDs needed to be implanted to save 1 life). Following this, investigators began to explore the use of MTWA to find a low-risk subset of “MADIT-like” patients who might have event-free survival without protection by an ICD. Studies by Hohnloser and Bloomfield pointed out the very high negative predictive value of MTWA in this population and proposed that patients with negative MTWA results (positive and indeterminate results were grouped as “non-negative” or “abnormal”) might safely avoid ICD implantation.39,40 Chow et al studied 768 patients with ischemic cardiomyopathy and, by using propensity analysis, determined that ICD implantation offered a much higher mortality benefit in patients with non-negative MTWA results.41
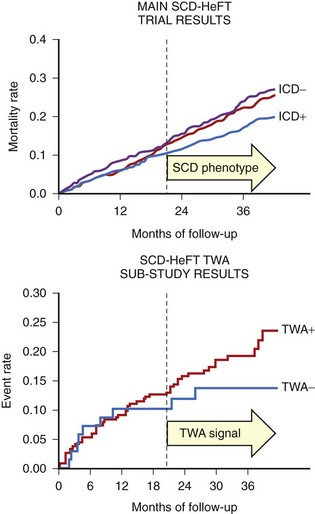
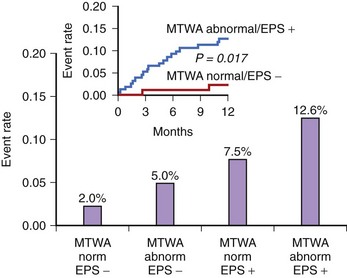
In the Alternans Before Cardioverter Defibrillator (ABCD) trial, a 95% negative predictive value of MTWA at 1 year was comparable with the negative predictive value of invasive electrophysiological testing; at 2 years the negative predictive value of MTWA was not as high.42 This study showed that (1) noninvasive MTWA testing can be used to improve primary prevention (separating higher risk patients from lower risk patients) without the risk or cost associated with invasive electrophysiological testing, (2) risk stratification may need to be repeated periodically because the myocardial substrate changes over time, and (3) the value of MTWA plus electrophysiological testing was superior to that of either test alone, suggesting that multiple risk markers should be used in combination for optimal risk stratification (Figure 67-3). A subanalysis of the ABCD data showed that an abnormal MTWA predicted unstable ventricular arrhythmia, whereas a positive electrophysiological test predicted monomorphic VT.43
The Noninvasive Risk Assessment Early After a Myocardial Infarction (REFINE) study reaffirmed the advantages of combining multiple risk markers.44 In this study of patients who had an MI, impaired heart rate turbulence, abnormal MTWA, and low ejection fraction were combined to identify patients at high risk. Of note, this was true when patients were assessed 10 to 14 weeks but not 2 to 4 weeks after the MI. A study by Huikuri et al also showed that MTWA performed early after MI was not a useful risk predictor.45
In contrast to the studies that showed a high negative predictive value of MTWA in stable post-MI patients, the Microvolt T-Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients (MASTER) trial failed to show that MTWA status could predict ventricular tachyarrhythmic events (of which 90% were appropriate ICD therapies).46 Published in the same year, the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) alternans substudy, which randomized both patients with MI and those with nonischemic cardiomyopathy to ICD, amiodarone, or placebo therapy, reported that MTWA testing did not predict arrhythmic events or death.47 As pointed out in the editorial that accompanied this report, however, the survival benefit of the ICD in SCD-HeFT only emerged between 18 and 24 months after randomization, at exactly the time that a difference in event-free survival became apparent in the populations with normal and abnormal MTWA results (Figure 67-4).48 Hohnloser showed that in prospective clinical trials evaluating MTWA as a risk predictor, trials with a low rate of ICD implantation showed a consistently high negative predictive value of MTWA.49 In contrast, trials that used ICD therapies as a surrogate endpoint for SCD found MTWA to be less useful. Hohnloser concluded that MTWA has a high negative predictive value for SCD but that ICD therapies are an unreliable surrogate endpoint for SCD.
Microvolt T-Wave Alternans in Patients with Other Types of Cardiomyopathy
Several studies have shown MTWA to be a valuable risk predictor in patients with heart failure.25,50 These studies, which combined patients with ischemic cardiomyopathy and those with nonischemic cardiomyopathy, showed a high negative predictive value of MTWA, as did additional studies that focused only on patients with nonischemic dilated cardiomyopathy.36,51,52 The usefulness of MTWA for predicting the risk of ventricular tachyarrhythmias in the nonischemic cardiomyopathy population stands in contrast to the insensitivity of other risk stratifiers, including invasive electrophysiological testing. However, a recent study of 286 patients with ejection fraction of 35% or less (ischemic and nonischemic cardiomyopathies) raised questions about the adequacy of MTWA for identifying a low-risk subgroup.53 In this study, the negative predictive value of MTWA for 2-year total mortality rate was only 90%. It should be noted, however, that patients in this study had nonsustained VT, syncope, or both and therefore had a higher baseline risk than most primary prevention populations.
Microvolt T-Wave Alternans as a Marker of Risk in Patients with Preserved Ejection Fraction
Recently, Ikeda et al showed that MTWA can identify a high-risk subgroup among patients with well-preserved ejection fraction after myocardial infarction (MI).54 MTWA has also shown promise as a risk predictor in hypertrophic cardiomyopathy.55,56 In contrast, MTWA has not been helpful as a risk discriminator in congenital long QT syndrome (LQTS) or in Brugada syndrome.57–59 Thus MTWA appears to be of value in disease states associated with structural heart disease.
Directions for Further Study
MTWA has been well established as a valuable predictor of risk for SCD among patients with structural heart disease. However, significant questions remain. MTWA is a dynamic phenomenon that is affected, for example, by heart rate and by β-blockade. It is not known how early MTWA should be measured after MI for the best long-term risk prediction and how often MTWA testing should be repeated. Because a patient’s arrhythmogenic substrate may change over time (because of scar maturation, ischemic events, changes in left ventricular function), serial MTWA testing will likely add value to risk assessment. The value of MTWA measurement during dual-chamber pacing or during pharmacologic heart rate elevation requires further study, as does the modified moving average technique. The magnitude of MTWA and its onset heart rate may contribute additional prognostic information to the traditional MTWA test. The usefulness of MTWA as a prognostic tool in lower risk populations (such as patients with preserved left ventricular function after MI) has only begun to be explored.54 Finally, genetic polymorphisms that underlie susceptibility to MTWA (and to ventricular arrhythmia) require exploration.
Signal-Averaged Electrocardiography
Gioia Turitto, Raushan Abdula, and Nabil El-Sherif
Signal-averaged electrocardiography (SAECG) is one of several proposed methods for noninvasive risk stratification for SCD. The term signal-averaged electrocardiography refers to techniques for improving the signal/noise ratio, thus allowing analysis of signals that are too small to be detected by routine measurement. Among such signals are those arising from areas of slow and inhomogeneous conduction in diseased ventricular myocardium (usually referred to as late potentials [LPs]). These potentials are small because the activation front is slow and fractionated, the mass of tissue undergoing depolarization is small, or both. LPs are of clinical relevance because they may identify a substrate for re-entrant ventricular excitation.60
Important technical advances in the field of SAECG in the early 1980s included the introduction and refinement of filtering techniques, the selection of bipolar orthogonal leads, and their combination into a vector magnitude for maximal sensitivity as well as the use of computer algorithms to identify QRS offset and provide numeric values for signals in the terminal part of the QRS.61 In 1991, a Task Force of the American College of Cardiology (ACC), the American Heart Association (AHA), and the European Society of Cardiology (ESC) published standards for the acquisition and analysis of LPs and attempted to define clinical indications for the SAECG.62 The Task Force original recommendations were updated by an ACC Expert Consensus Document.63 The recommended applications consisted of risk stratification for future arrhythmic events and SCD in survivors of MI and prediction of malignant ventricular tachyarrhythmias in patients with coronary artery disease (CAD) and syncope or asymptomatic nonsustained VT. Other groups of patients with organic heart disease in whom SAECG has been used for risk stratification for SCD include patients with idiopathic dilated cardiomyopathy, hypertrophic cardiomyopathy, right ventricular cardiomyopathy. More recently, the prognostic value of SAECG was examined in a statement prepared by a Joint ACC/AHA/Heart Rhythm Society Writing Group on noninvasive risk stratification techniques for identifying patients at risk of SCD.64 This Expert Consensus Document acknowledged that an abnormal SAECG may identify patients with prior MI at risk of SCD. Given its high negative predictive accuracy, SAECG could be useful for the identification of low-risk patients. However, the document noted that routine use of SAECG to identify patients at high risk for SCD is not adequately supported by existing data and further studies would be required to assess the usefulness of this test.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree