Introduction
The complexity of the diagnostic and clinical management aspects of thoracic aortic aneurysmal disease is reflected in Sir William Osler’ s famous aphorism “there is no disease more conducive to clinical humility than aneurysm of the aorta.”1 Shennan drew attention to a particularly lethal aortic pathology – aortic dissection – in his 1934 monograph published by the Medical Research Council.2 It was, however, only with the advent of successful surgical therapies introduced by DeBakey and Cooley in the 1950s3,4that medical attention in these conditions moved from curiosity to cure.
Joyce noted in his seminal observational series of aortic aneurysmal disease in Olmsted County in 1960 that the advent of a realistic surgical option rendered an understanding of the natural history of the disease critical to assessment of the relative risks and benefits of intervention.5 In this series of patients seen at the Mayo Clinic between 1945 and 1956 with thoracic aortic aneurysmal disease, 50% were dead within 5 years of diagnosis. Even then, he and his co-authors identified aortic diameter as a powerful predictor of rupture, the parameter which has dominated our understanding since. Indeed, it is remarkable that they focused on 6 cm as a particularly critical dimension, with less than 40% of patients presenting with aneurysms greater than 6 cm in diameter alive at 5 years. Follow-up studies from Olmsted County portrayed an even worse prognosis, with only 13% of 72 patients individuals presenting with thoracic aneurysms between 1951 and 1980 alive at 5 years.6 This may not be surprising as over half of the patients in the latter series presented with rupture as their first indication of the disease, given the limited diagnostic modalities of the day. Perhaps as a reflection of improved radiologic techniques, the apparent natural history would appear to have improved, with more recent studies from the same institution reporting a 20% risk of rupture at 5 years for thoracic aneurysms.7 At the same time, there was an apparent threefold increase in the incidence of thoracic aortic aneurysms. Incidentally, given our dependence on imaging modalities for detection of aortic disease, this phenomenon of interplay between diagnostic technology and apparent clinical behavior continues to impact our understanding of conditions of the aorta today.
Just as diagnostic modalities have improved over the last several decades, so have therapeutic options. Beginning with DeBakey’ s introduction of Dacron prosthetic grafts for aortic replacement,8 landmark improvements have included Bentall’ s introduction of the technique for root replacement9 as well as Griepp’ s introduction of profound hypothermia and circulatory arrest for the management of aneurysmal disease of the aortic arch.10 Today aortic root replacement can be accomplished with a mortality rate less than 5% in experienced hands11 and arch replacement with similar risk under elective circumstances.12,13 Using appropriate adjuncts, the risks have also dropped dramatically in recent years for elective repair of descending thoracic14 and thoracoabdomional aortic aneurysms15,16 in centers of excellence. Most recently, endovascular technologies have been introduced for repair of descending thoracic aortic aneurysms, again challenging us to find the balance between risks of the intervention and the natural history of the disease. Equally the advent of valve-sparing aortic root repair has altered the risk/benefit analysis and threshold for recommending prophylactic aortic surgery.17
By far the majority of aortic conditions presenting in adults requiring surgical intervention are aneurysmal in nature. While coarctation and even interrupted aortic arch may present in adults, these are uncommon and are perhaps most fruitfully discussed in the context of adult congenital heart disease. The majority of aneurysms are “degenerative” in nature, in contrast to traumatic pseudoaneurysms and chronic dissections. By definition, the wall of a pseudoaneurysm lacks all three layers of the normal aortic wall – intima, media and adventitia – and accordingly chronic dissections fall into this classification as well, although they are seldom referred to as such. Postsurgical anastomotic pseudoaneurysms may also occur, although they are remarkably uncommon.18
The term “degenerative” is applied to aneurysms associated with a wide variety of conditions ranging from mundane to exotic. Chronic hypertension, particularly when combined with tobacco abuse, accounts for the majority of aneurysms of the ascending aorta, with connective tissue diseases of defined or undefined type accounting for a smaller but significant fraction.19 Giant cell arteritis is increasingly recognized as a cause of aneurysmal disease, particularly among women.20 Finally, ascending aortic aneurysms associated with bicuspid aortic valve disease are also generally considered “degenerative” and account for a considerable fraction of ascending aneurysms coming to surgery today.
The distinction between aortic ectasia and true aneurysmal dilation is subject to some degree of interpretation. Nomograms depicting normal aortic dimensions versus body surface area may be useful,21 particularly in pediatric populations; however, there is no bright line between enlargement and aneurysm. Furthermore, changes in body mass, which may occur with age, can certainly influence these assessments in the adult. Perhaps a more practical definition of aneurysm as suggested by Crawford is enlargement to greater than twice the diameter of the adjacent normal aorta.22
Full characterization of aortic disease must also include a description of the location and extent of the aneurysm itself. This should ideally include distinction between dilation of the ascending aorta and of the root. Either or both may be enlarged. Accordingly, in our institution, we have adopted a classification scheme as depicted in Figure 67.1. Unfortunately this distinction is not always made in the literature. Accordingly there is sometimes confusion over what best to do with a patient having enlargement of the sinuses but not the ascending aorta (Fig. 67.2) when guidelines refer to the ascending aorta specifically. Safihas proposed a classification scheme for aneurysms of the descending thoracic aorta as well (Fig. 67.3), and has demonstrated its correlation with operative risk.15 Thoracoab-dominal aneurysms in turn are conventionally classified according to Crawford’ s schema as shown in Figure 67.4.23
Figure 67.1 Although it is widely recognized that bicuspid aortic valve is associated with ascending aortic aneurysm, in point of fact, there are at least three phenotypes which should be distinguished. The most common is a supracoronary aneurysm confined to the ascending aorta. We refer to this as a Type I aneurysm. The Type II aneurysm demonstrates effacement of the sinotubular junction with generalized enlargement of the ascending aorta and root. This pathology more commonly demands complete root replacement surgically. Type I in contrast can be managed with a separate valve and graft. The least common is Type III with aneurysmal dilatation of the root reminiscent of Marfan syndrome. The ascending aorta can, in fact, be of entirely normal diameter with quite significant enlargement of the sinuses.
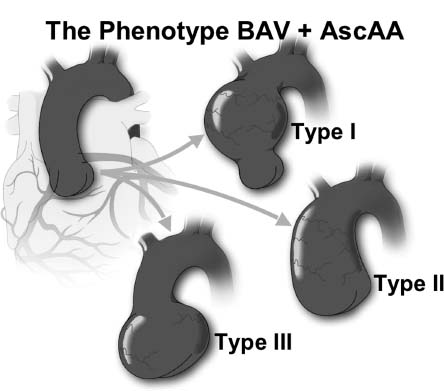
Figure 67.2 This sagittal CT scan demonstrates a Type III aneurysm with significant enlargement of the sinuses of Valsalva but a relatively normal ascending aorta.
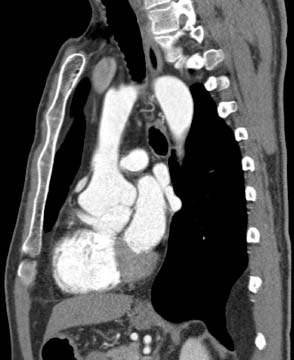
Figure 67.3 Safihas defined three types of descending thoracic aortic aneurysms with, from right to left, Type A aneurysms involving the upper descending thoracic aorta alone, Type B aneurysms involving the lower descending thoracic aorta and, Type C aneurysms involving the entire descending thoracic aorta. The risks associated with surgical intervention are correspondingly associated with the extent of the disease.
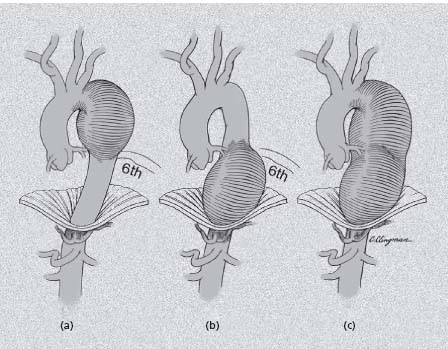
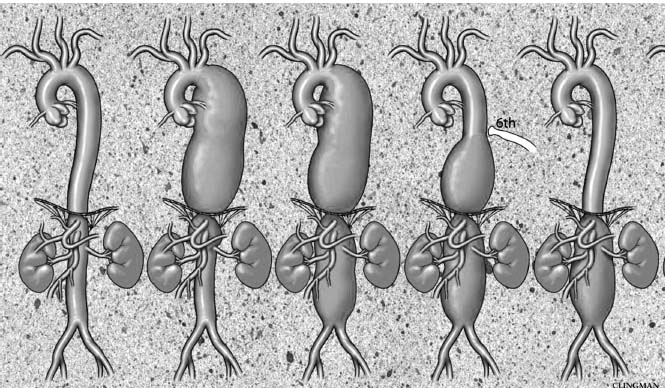
Figure 67.4 The Crawford classification of thoracoabdominal aneurysmal disease includes Type I aneurysm with involvement of the entire descending thoracic aorta and upper abdominal aorta including the origins of the visceral vessels, a Type II aneurysm involving the descending thoracic aorta and entire abdominal aorta, Type III – an aneurysm involving the lower descending thoracic aorta and abdominal component and Type IV – perivisceral abdominal aortic aneurysm involving the origins of the visceral vessels but sparing the majority of the descending thoracic aorta.
The location and extent of aortic dissection are critical in clinical decision making as well. Acute aortic dissection represents a mechanical failure of the aortic wall with the propagation of a plane of dissection within a weakened media creating a “double-barrel” aorta with a channel of flow into a true and false lumen. Uncommonly, the latter may thrombose completely; more often the false lumen remains at least partially patent and is actually larger than the true lumen. In some instances the false channel may compress the true lumen, causing a pseudo-coarctation, or in the extreme may cut off blood flow to end-organs, causing a malperfusion syndrome. Originally classified by DeBakey into three types (Fig. 67.6),4 a simpler if somewhat less informative system proposed by Shum-way’s group (Fig. 67.6)24 has become more popular, particularly among non-surgeons. In the latter system, those dissections involving the ascending aorta, including both DeBakey type I and II, are classified as “Stanford type A” which is easily remembered (“A” for “ascending”) and is of practical value as the immediate clinical management is the same. Those not involving the ascending aorta (DeBakey type III) are by elimination “Stanford type B.”
Figure 67.5 The pathogenesis of aortic dissection is thought to originate with an intimal tear which allows blood entry into the media with propagation of the dissection distally. In some instances, the false channel will thrombose resulting in a “noncommunicating dissection.” It is currently held that intramural hematoma begins with rupture of the vasorum progressing to a clotted hematoma. In some instances, that hematoma may rupture into the lumen allowing dissection to form.
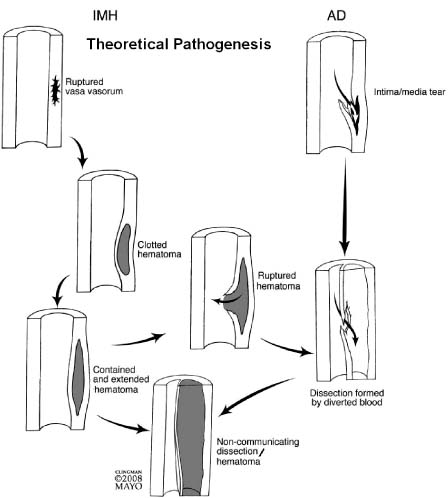
Figure 67.6 The classification of aortic dissection may be either the DeBakey or Stanford classification. From left to right, the most common is the DeBakey Type I or Stanford Type A dissection involving the entire aorta. DeBakey Type II dissection involves the ascending aorta alone. Because it involves the ascending aorta, it is also classified as a Stanford Type A. The DeBakey Type III dissection involves the descending thoracic (III-A) or thoracoabdominal (III-B) aorta and may also be termed a Stanford Type B dissection.
Aortic dissections are also the principal causes of “acute aortic syndromes,” a term coined by Vilacosta and Roman in reference to clinical presentation of a variety of aortic pathologies intended to draw attention to these entities in contrast with the much more common causes of chest pain – “acute coronary syndromes.”25 Unfortunately, the former continues frequently to be mistaken for the latter even in today’ s age of advanced imaging modalities.26 Given the opposing aims of therapies for each – anticoagulation versus surgical intervention – this misdiagnosis can have tragic consequences.
Other causes of acute aortic syndrome include symptomatic thoracic aortic aneurysm (a relatively uncommon condition) and the so-called “variant forms” of acute dissection – intramural hematoma (IMH) and penetrating atherosclerotic ulcer (PAU). By definition, the term intramural hema-toma refers to the presence of hematoma within the wall of the aorta without apparent intimal disruption as described in 1920 by Krukenberg.27 This definition was created at a time when the diagnosis of intramural hema-toma was made at autopsy or, somewhat more recently, at open surgical intervention. In the current era, the diagnosis of these conditions is most often made radiologically and accordingly a distinction between IMH and so-called “non-communicating dissection” with complete thrombosis of the false lumen can be difficult. Indeed, one could argue that such a distinction is impossible, as it relies upon the demonstration of absence of a disruption. Lack of active flow communication is more straightforward, of course, and from a practical standpoint may be more important as IMH and non-communicating aortic dissection with thrombosis of the false lumen likely behave very much alike.
Penetrating atherosclerotic ulcer as a distinct entity was most clearly defined by Stanson and colleagues in 1986.28 Penetrating atherosclerotic ulcers represent intimal disruptions with extension into the medial layer. They most often occur in the heavily atherosclerotic descending thoracic aorta with a propensity to arise in the distal descending thoracic aorta. Typically they are surrounded by at least some element of IMH (although if one is adherent to Kruken-berg’s definition above one would question the use of this term). Penetrating atherosclerotic ulcers are most often discussed in the context of acute aortic syndromes, although a significant percentage are identified as incidental findings during radiologic studies for other reasons.29 The principal radiologic challenge is differentiating this entity from heavy intraluminal thrombus with surface irregularities.
A variety of congenital conditions can also cause aneurysms of the aorta. Among these the most common is dilation of the origin of an aberrant subclavian artery (Fig. 67.7). Most often this is an aberrant right subclavian artery in the presence of a left aortic arch although the converse may occur as well. The origins of such vessels are often somewhat dilated and again as noted above, the distinction between a diverticulum of Kommeral and true aneurysm is somewhat subjective. The indication for intervention is as often dysphagia lusoria from compression of the esophagus as it is the actual dimensions of the structure.
Figure 67.7 Aberrant origin of the left subclavian artery from a right aortic arch with a diverticulum of Kommeral.
As noted above, both the estimated incidence and apparent natural history of thoracic aortic aneurysmal disease depend to an extraordinary degree on imaging technology, in terms of both technical resolution and clinical application, since most aneurysmal disease is asymptomatic until disaster strikes. Still, in most modern studies the incidence of thoracic aortic aneurysms is estimated at between 8 and 12 per 100,000 person-years. In the Olmsted County study mentioned above, Clouse and colleagues estimated the incidence of thoracic aneurysms in that population to be 10.4/100,000 person-years.7 Perhaps the most comprehensive study of thoracic aortic aneurysmal disease to date was reported by Olsson in 2006, with unique tracking of the population of Sweden over a 16-year period.30 This study encompasses a population of 8.7 million. Thoracic aortic aneurysms greater than 5 cm in diameter, true dissections and intramural hematomas were tracked. The incidence appeared to gradually rise over the time of the study with approximately 16.3 males/100,000 person-years and 9.1 females/100,000 person-years diagnosed in 2002. As the apparent incidence rose, prognosis improved with an increasing frequency of surgical procedures performed and a declining operative mortality.
With regard to acute aortic syndromes and aortic dissection, in another Olmsted County study, Clouse and colleagues estimated the incidence of aortic dissection at 2.9 per 100,000 persons per year.31 Meszaros has estimated the incidence in a Western European study to be 3.5 per 100,000 persons per year.32 Two-thirds of these dissections involve the ascending aorta.33 Intramural hematomas are generally thought to represent approximately 5 – 10% of acute aortic syndromes34 whereas PAU are far less common. The latter two conditions tend to occur in patients of somewhat more advanced age.
The natural history of thoracic aortic aneurysmal disease is progressive. The most commonly cited predictor of behavior as identified by Joyce in all subsequent studies is aortic diameter, with risk rising particularly beyond 6 cm.5,35 In the study by Clouse et al, the rupture risk for aneurysms less than 4 cm in diameter was negligible, while that for aneurysms between 4 and 5.9 cm was 16% and that for aneurysms 6 cm or larger was 31%.7 Other factors enter play as well, although less well recognized perhaps. Clouse identified female gender and advanced age as risk factors for rupture.7 Juvonen and Griepp performed a sophisticated analysis of risk factors for rupture of thoracoabdomi-nal aneurysms,36 confirming these risk factors as well as chronic obstructive pulmonary disease. Not surprisingly, hypertension has been identified as a risk factor as well.37
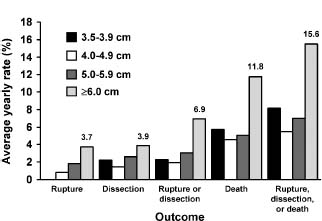
The most comprehensive analysis of the impact of diameter on rupture risk has been performed at Yale University through the efforts of Elefteriades and Rizzo using their thoracic aortic aneurysm database. A number of important publications have derived from this work. Coady identi-fied a hinge point for rupture of the ascending aorta at 6 cm while that for descending thoracic aorta was 7.2 cm.38 As intervention at this dimension would be at the expense of a significant number of ruptures, most surgeons have adopted 5.5 cm for the ascending aorta and 6.5 cm for the descending thoracic aorta as criteria for intervention (Class I, Level C). Subsequent work has demonstrated that the annual risk of aortic catastrophe, taking rupture, dissection or death as the composite endpoint, rises to 15.6% for aneurysms greater than 6 cm in diameter as shown in Figure 67.8.37 The correlation is even stronger when the diameter is indexed as body surface area: an indexed dimension of 2.75cm/m2 is associated with a risk of 4% per year while that greater than 4.25 cm/m2 is associated with a risk of approximately 20% per year.39 Unfortunately, the weakness of these data is that they are derived from a surgical database, and therefore may be somewhat biased toward malignant behavior as in any such database the cases entered represent those referred for consideration, in contrast to population-based studies.
Figure 67.8 The risks associated with aortic aneurysms of various diameter as determined by Davies et al. from the Yale University Thoracic Aortic Database.
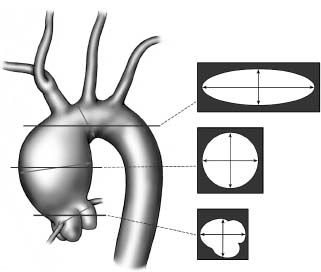
Implicit in these guidelines is the accurate measurement of aortic dimensions. Whether by echocardiography or computed tomography, the dimensions measured depend upon the selection of the optimal image with maximal dimensions while appropriately placing the measurement cursor normal to the long axis of the vessel. As shown in Figure 67.9, oblique images will provide deceptively large measures if one is not cautious. Surgeons therefore speak of using the smallest measured diameter on the image with the largest dimensions. On the other hand, asymmetric or saccular aneurysms should be measured in the longer dimension. In our view, the advent of three-dimensional reconstructions of aortic images has proven very beneficial. It should also be noted that the sinuses of Valsalva are always somewhat larger than the ascending aorta and the majority of studies reporting ascending aortic dimensions do not distinguish whether they were evaluating the ascending aorta or root. In all likelihood, the majority are referable to the ascending aorta. Still, we apply these dimensions even when the dilation is largely in the root. In particular in the setting of Marfan syndrome and some familial thoracic aortic aneurysms, the ascending aorta itself may be remarkably normal in dimension with almost all the dilation in the sinuses themselves. Therefore, one may be just as readily lulled into a false sense of security with a falsely low aortic dimension if only those dimensions in the ascending aorta are considered.
Figure 67.9 The true diameter of the aorta at any given level as derived by 2-D axial imaging will be the smallest diameter as measured on the image at that level. Oblique images will yield an oval, of which the smallest diameter is most accurate assuming a cylindrical structure. Three-D reconstructions permit more accurate assessments measuring the diameter normal to the axis of blood flow.
It is likely that the risk of rupture can be reduced through the administration of antihypertensive medications (Class IIa, Level B). Among patients with Marfan syndrome, studies by Schor and colleagues40 and Rossi-Foulkes and co-workers41 have demonstrated the protective effect of beta-blockers or calcium antagonists respectively on aortic dilation and, in the former case, aortic complications. Yetman and associates have also utilized the angiotensin-converting enzyme enalapril in patients with Marfan syndrome demonstrating improved aortic distensibility, reduced aortic stiffness, and a smaller increase in aortic diameter.42 Exciting data from the Dietz laboratory at Johns Hopkins have also suggested that use of the angiotensin receptor blocker losartan may have a favorable impact on aortic remodeling as well.43
Although the details of the pathophysiologic basis of aortic aneurysmal disease are largely beyond the scope of this chapter, they may have an impact on clinical behavior. This is most obvious in the case of Marfan syndrome in which it is widely accepted that the risks of dissection and rupture are, size for size, greater than for aneurysms of other etiology.44 The association between mutations in the fibrillin gene and Marfan syndrome has been known for many years now.45 The pathogenesis was assumed to relate to the critical role of fibrillin in the extracellular matrix as the main component of the 10 – 20 nm microfibrils which associate with elastin in the media. Such mutations have been shown to increase susceptibility to proteolysis and, as a consequence, microfibril and elastin fragmentation. In the last several years, however, intriguing results from the Dietz laboratory have suggested a role for dysregulation of transforming growth factor beta (TGF-β) signaling in Marfan syndrome.46 Amino acid sequence homology identified in silico between fibrillin-1 and latent TGB-β proteins (LTBPs) suggested that alterations in fibrillin might impact TGB-β levels in the tissues due to decreased binding. Indeed, laboratory studies have shown that alterations in TGB-β signaling may lead to alterations in extracellular matrix homeostasis in the lung as well as the aorta. Subsequent studies have demonstrated an important role for TGB-β in other related aortopathies including mutations in the TGB-β receptor type I and II genes in the “Marfan syndrome II” phenotype and other familial thoracic aortic aneurysm syndromes.47–49 A particularly malignant form of such a familial syndrome is the recently defined Loeys–Dietz syndrome which may be secondary to mutations in TGF BRI or TGF BRII.50 Individuals so affected are particularly susceptible to widespread aneurysmal dilation early in life. The clinical significance of such findings is the potential for genetic testing, permitting prophylactic operative repair of the susceptible aortas of family members who may well be susceptible to dissection at lower aortic diameters.
The association between bicuspid aortic valve and ascending aortic dilation has long been recognized.51 There is mounting evidence that the aortopathy is primary, and not secondary to altered flow dynamics, as it may be present even with a functionally normal valve.52 Structural abnormalities including gross degenerative histologic abnormalities commonly referred to as “cystic medial necrosis,”53 thinning of elastic lamellae,54 smooth muscle cell apoptosis55 and increased matrix metalloproteinases56 have been observed in the associated aneurysmal aorta. While there is agreement that the risk of dissection is higher among patients with a bicuspid valve,57 the relationship between dissection and aortic diameter is less so, with no studies thus far documenting a higher risk of aortic complications at a given dimension than other aortic dilations. Accordingly, while the presence of documented Marfan syndrome or another familial syndrome can be cogently argued as a rationale for predicting a worse outcome and therefore more aggressive approach to management, the same cannot as clearly be said for bicuspid disease. Having said this, a small study by Svensson and colleagues substantiates the notion that patients with bicuspid disease may dissect at dimensions less than 5 cm.58 Recent data from the IRAD database demonstrate that this may be true even in patients with trileaflet valves,59 although such studies look only at the numerator and accordingly cannot address actual risk for the population as a whole. Since a very large number of patients have an aortic diameter less than 5.0 or 5.5 cm, the occurrence of dissections among these individuals may take place at a very low incidence – potentially far less than the risk of aortic replacement surgery. The argument for treating patients with bicuspid disease differently with a specifically lower threshold for replacement is therefore weakened. Nonetheless, it is well documented that the ascending aorta may continue to dilate even after aortic valve replacement in the setting of bicuspid disease.60,61 Accordingly, a number of investigators have advocated prophylactic replacement of the ascending aorta at the time of bicuspid valve repair or replacement should it exceed 4.5 – 5 cm (Class IIb, Level C). These criteria are generally accepted.
The diagnostic modalities employed in evaluation of aortic disease are similar regardless of the etiology of the condition. By far the most commonly employed technology is computed tomography. This is in part due to its widespread availability. Image acquisition is also less operator dependent although interpretation still requires special expertise. Indeed, the majority of thoracic aortic aneurysms are asymptomatic and are identified at the time of CT scanning for other reasons. CT scanning is also the most common first modality employed in the diagnosis of thoracic aortic dissection.33 Frequently such a study is performed for the purpose of ruling out pulmonary embolism in the patient with acute-onset chest pain without ECG changes or enzymatic evidence of myocardial infarction. It should be noted that since aortic aneurysmal disease is commonly multifocal,22,62 complete imaging of the entire thoracic and abdominal aorta should be performed in all patients with a diagnosis of thoracic aortic aneurysm.
Echocardiography is also commonly employed in the evaluation of patients with thoracic aortic disease. Indeed, many ascending aortic aneurysms are discovered incidentally during echocardiography for suspected valvular conditions. This highlights the importance of imaging of the ascending aorta, arch to the extent possible, and descending thoracic aorta as a standard part of routine transthoracic and transesophageal echocardiography. Transesophageal echocardiography is the second most common modality used in the diagnosis of acute dissection and is particularly helpful in that context for evaluating the function of the aortic valve in that setting.33
Magnetic resonance imaging is less commonly performed as the initial diagnosis test.63 It has a greater role perhaps in serial follow-up of patients with aneurysmal disease as it does not subject the patient to radiation. In the case of a young individual, the amount of radiation delivered by annual or biannual CT scanning for follow-up of thoracic aortic aneurysm can be quite considerable. In many cases, MRI is selected as the technique of choice for serial exams. Most surgeons find these images cumbersome, however, and prefer CT scanning.
Management of thoracic aortic aneurysmal disease is dependent upon the location within the aorta, the etiology of the aneurysm and the acuity of its presentation. The thoracic aorta is itself a more complex structure than might be presumed. Far from being a simple tube, it has significant elastin content that serves a “Windkessel function” of storing energy in systole and returning it in diastole.64 This improves coronary artery blood flow proximally, and likely helps to distribute forces within the vascular tree more effectively. Elastin content varies along the length of the aorta, with the highest content proximally. Given very different forces65 and mechanical properties,66 it should come as no surprise that the behavior of pathologic entities such as classic aneurysm, IMH or acute dissection varies according to location. The etiologic basis of the aneurysmal condition may also play a role in determining the optimal management. This is particularly true for individuals with genetic causes of aneurysmal disease including Marfan syndrome, familial thoracic aortic aneurysm, and bicuspid aortic valve in whom the mechanical properties of the aorta itself appear altered.67 While our understanding of these conditions is still in its adolescence, if not infancy, in general a more aggressive posture is adopted towards those aneurysms and dissections associated with an underlying genetic cause.68 Finally, the acuity of presentation is an important determinant of the management strategy and will be reflected below by our separation of the management strategies according to this parameter.
Acute aortic syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


