Clinical Evaluation, Diagnosis, and Staging of Lung Cancer
INTRODUCTION
Patients who present with suspected lung cancer require a detailed clinical evaluation followed by noninvasive testing and invasive procedures to establish both the histopathologic diagnosis as well as disease stage. Historically, great emphasis has been placed upon the differentiation of small-cell lung cancer (SCLC) from non–small-cell lung cancer (NSCLC). SCLC, which accounts for 14% of bronchogenic carcinomas, is histologically and clinically distinct from NSCLC.1 NSCLCs have been traditionally regarded as a fairly uniform group of cancers. However, it has become increasingly evident that NSCLCs are comprised of clinically, pathologically, and molecularly diverse tumors that respond to different therapeutic agents based on specific histologic phenotypes and molecular characteristics.
The clinician evaluating the patient with suspected lung cancer must take into account several factors, including the likelihood of SCLC versus NSCLC, probability of metastatic disease, comorbid illness and functional status, presence of paraneoplastic syndromes, and treatment preferences of the patient. Good decision making regarding appropriate diagnostic test selection must incorporate: (1) careful assessment of pretest probabilities based upon clinical evaluation and initial radiographic features, and (2) understanding of specific test characteristics. The main objectives of the diagnostic and clinical staging evaluation are to obtain adequate tissue to establish the histopathologic diagnosis and, when indicated, molecular characterization of the tumor, and to ascertain the extent of disease to determine candidacy for specific therapies. These twinned goals should be accomplished in the safest, least invasive, and most cost-effective manner possible.
INITIAL CLINICAL EVALUATION
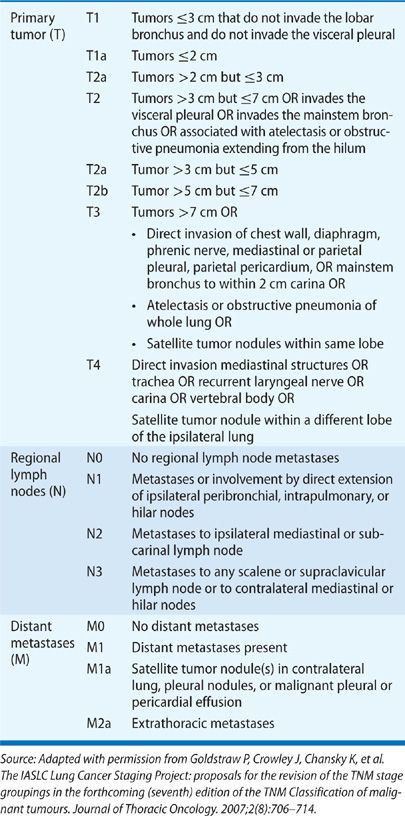
A detailed history and physical examination are of key importance in assessment of the patient’s overall health status and medical appropriateness for specific therapies.2 Certain comorbid conditions may reduce therapeutic options. Limited cardiopulmonary reserve may preclude surgical intervention. A thorough history can also assist a physician in determining overall extent of disease. At the time of presentation, it is most useful to consider the clinical stage using the International Association for the Study of Lung Cancer (IASLAC) 7th edition of the tumor, node, and metastasis (TNM) classification system (Table 112-1); pathologic stage is established only after surgical resection.3
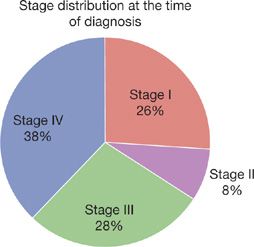
Only 7% to 13% patients are asymptomatic at the time of initial diagnosis as lung cancer is usually a diagnosis made during its later stages.4,5 A recent national cancer database survey of patients diagnosed from 1998 to 2006 demonstrates that the majority of patients have either locally advanced or metastatic disease at the time of diagnosis. Only 26% of patients have stage I disease and 8.3% have stage II, whereas 27.6% have stage III disease and 38.1% have metastatic disease (Fig. 112-1).6
Figure 112-1 Stage distribution at the time of diagnosis.
Patients often have had symptoms for months prior to diagnosis.7,8 Symptoms, particularly those associated with localized disease such as cough, wheezing, and dyspnea, are often attributed to comorbid illnesses by both patients and their physicians. In one study in the United Kingdom of newly diagnosed lung cancer patients, symptom duration ranged from 4 to 24 months with a median of 12 months.9 In one of the earliest series describing the relationship of presenting symptoms to stage and prognosis, about a third of patients (27%) presented with symptoms related to primary tumor only (Table 112-2), another third (34%) presented with nonspecific symptoms concerning for metastatic disease, and the final third (32%) presented with symptoms attributable to a distant metastatic site.10 Only 6% were asymptomatic.
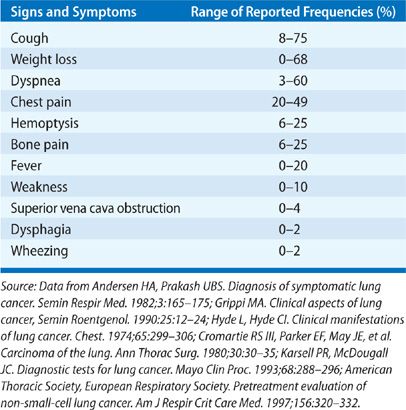
Local symptoms can include cough, hemoptysis, dyspnea, chest pain, and wheezing.5,11 Cough is the most frequently encountered presenting symptom and can be the result of endobronchial or parenchymal involvement by the primary tumor, regional lymph node enlargement, or postobstructive complications. Hemoptysis is the symptom that typically results in the most expeditious evaluation; about 5% of patients with hemoptysis and a normal chest x-ray will ultimately be diagnosed with lung cancer.12 Chest discomfort and pain may indicate pleural involvement or direct tumor invasion of the chest wall or thoracic cage. Hoarseness, which can occur in up to 5% lung cancer patients at presentation, can be concerning for laryngeal nerve palsy, which can occur in 2% to 18% of lung cancer patients.5 This is more commonly seen with left-sided tumors, which can involve the recurrent laryngeal nerve as it loops under the aortic arch.11 Lung cancer accounts for up to 75% of all cases of superior vena cava (SVC) syndrome due to local compression or invasion.13 Signs and symptoms of SVC syndrome include swelling of the face, neck, upper torso and arms, and dyspnea. Up to 4% of patients with NSCLC and 10% of patients with SCLC can develop SVC syndrome. Dysphagia can suggest extrinsic compression of the esophagus due to bulky mediastinal disease, particularly involving the subcarinal lymph node, or it can result from direct esophageal invasion. Central tumors such as squamous cell carcinoma may cause airway obstruction resulting in a localized or unilateral wheeze, atelectasis, or postobstructive pneumonia.14,15 Cancers arising in the lung apex (superior sulcus tumors) can present with the Pancoast syndrome, an assortment of clinical manifestations due to invasion or compression of local structures. Characteristic signs and symptoms include shoulder pain due to invasion of the brachial plexus and/or ribs and vertebrae, upper extremity weakness and paresthesias, and Horner syndrome (unilateral ptosis, meiosis, and lack of facial sweating on the involved side) due to compression or invasion of the sympathetic chain.16
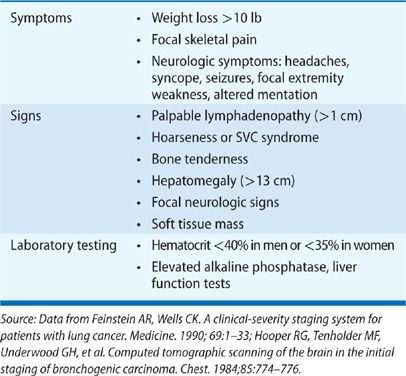
Focal symptoms of metastatic disease are fairly common at presentation: 25% of patients present with bone pain, 20% of patients present with symptoms associated with bulky mediastinal adenopathy such as cough and hoarseness, and 10% of patients have neurologic symptoms that may include headache, nausea and emesis, seizures and/or mental status changes at the time of diagnosis.5,11 Lung cancer accounts for 70% of the cancers that first present as symptomatic brain metastases.17 The presence of nonspecific constitutional symptoms (weakness, weight loss, fever, and anorexia) is worrisome for metastatic disease; often, patients with hepatic metastases present with symptoms of weakness and weight loss. Standard laboratory testing should include a complete blood count as well as chemistries that include liver function testing. Anemia (hematocrit less than 40% in men and less than 35% in women) can indicate higher likelihood of metastatic disease. Similarly, elevated liver function tests can be worrisome for hepatic metastases.
Hooper et al.18 have enumerated a standard set of clinical features found to be associated with metastatic disease (Table 112-3). Systematic review suggests that absence of all of these features makes metastatic disease highly unlikely with a negative predictive value exceeding 90%.19,20
TABLE 112-3 Features of a Standardized Evaluation for Metastatic Disease5
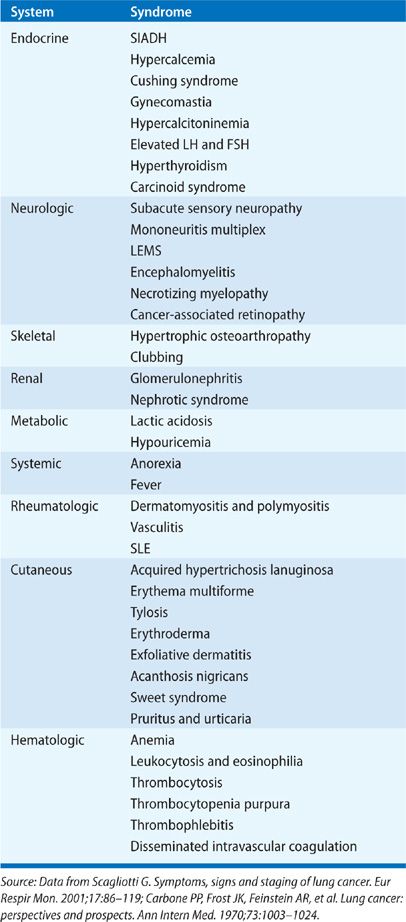
Initial clinical evaluation of the patient with suspected lung cancer should also include an assessment for paraneoplastic syndromes (Table 112-4), clinical disorders associated with malignant disease that are not directly attributable to the physical effects of the tumor. They may be seen in up to 10% patients with lung cancer.5 The most commonly recognized paraneoplastic syndromes are hormonally mediated; tumor cells secrete hormones or hormonal analogs. The syndrome of inappropriate antidiuretic hormone (SIADH) is seen in 10% to 45% of patients with SCLC and 0.7% to 1% of patients with NSCLC (squamous cell and adenocarcinoma) although symptoms related to hyponatremia are present in just 1% to 5% of patients.21,22 Hyponatremia is associated with decreased survival in SCLC.23 Ectopic production of adrenocorticotropin hormone (ACTH) can be found in up to 50% of patients with lung cancer, but true Cushing syndrome is seen in only 1% to 5% of patients with SCLC.5,21 Ectopic Cushing syndrome is also associated with a poor prognosis in SCLC, in part related to an increased susceptibility to opportunistic infection in patients given chemotherapy.21,24–26 Hypercalcemia is seen in 2% to 6% of all lung cancer patients at presentation, and in up to 10% to 25% during their clinical course.5,21 Etiology of hypercalcemia can be due to direct tumor effect on local osteolytic activity when there are bony metastases. However, in a substantial fraction of patients, typically those with squamous cell histopathology and less commonly in SCLC, hypercalcemia is due to tumor cell–elaborated parathyroid hormone related peptide (PTHrP).
TABLE 112-4 Paraneoplastic Syndromes Associated with Lung Cancer5
Digital clubbing and hypertrophic osteoarthropathy (HOA) are two paraneoplastic syndromes of the musculoskeletal system commonly associated with NSCLC. Clubbing manifests as enlargement of the terminal tufts of the fingers and toes. It results from the proliferation of connective tissue beneath the nail matrix and can be seen in up to 29% of patients with adenocarcinoma or squamous cell carcinoma histopathology.27 HOA, which occurs in fewer patients (<4%), presents as painful symmetric arthropathy, usually involving the ankles, wrists, and knees, and is caused by new periosteal bone formation at the distal long bones, resulting in vascular hyperplasia and edema. It is postulated that HPO occurs due to overexpression of vascular endothelial growth factor (VEGF).28
Other paraneoplastic syndromes associated with lung cancer occur due to the development of autoantibodies that can cause neurologic disease or rheumatologic disease. Dermatomyositis and polymyositis are autoimmune inflammatory myopathies characterized by progressive symmetrical proximal muscle weakness associated with various malignancies. Patients with dermatomyositis additionally may have Gottron’s papules, and a heliotrope rash with poikiloderma. In two series, lung cancer accounted for 17.4% and 18%, respectively, of new cancer diagnoses within the first year of diagnosis of dermatomyositis.29,30 The diagnosis can be confirmed by muscle or skin biopsy. Polymyositis is similarly associated with an increased risk of malignancy, with lung cancer accounting for 20% of newly diagnosed cancers during 5-year follow-up.29
There are a large number and variety of paraneoplastic neurologic syndromes (Table 112-3).31 Lambert Eaton myasthenic syndrome (LEMS) is one of the better defined; it occurs due to the development of autoantibodies against presynaptic voltage-gated calcium channels. These circulating autoantibodies can be found in up to 5% to 8% patients with SCLC although clinical LEMS occurs in only 1% to 3% of patients.32–34 Other paraneoplastic neurologic syndromes occur due to the presence of circulating neuronal autoantibodies (including anti-Hu, anti-Yo, and anti-Ri antibodies) and have a myriad of clinical manifestations.21 Up to 20% patients with SCLC have circulating anti-Hu antibodies although paraneoplastic syndromes do not occur in the majority.35 The related syndromes can include autonomic overactivity, corticocerebellar degeneration, brainstem encephalitis, limbic encephalitis, myelopathy, and peripheral nerve palsies. Autopsy studies have revealed that patients suffering from these syndromes have lymphocytic inflammatory infiltration in areas of the nervous system corresponding to their neurologic deficits.
While paraneoplastic syndromes manifest systemically, they are not always indicative of advanced malignant disease. Indeed, syndromes may regress with appropriate treatment of the tumor. Approximately 70% of patients with neurologic paraneoplastic syndromes have limited stage SCLC.
IMAGING EVALUATION
Following the initial intake evaluation, further noninvasive and invasive work-up should be undertaken with paired goals of (1) definitive establishment of histopathologic diagnosis and, when indicated, molecular characterization of the tumor, and (2) determination of disease stage in the most cost effective and least invasive manner possible. Given the myriad of diagnostic modalities available, it is often advisable that patients suspected of having lung cancer undergo this phase of the evaluation at an established multidisciplinary lung cancer center.
Unless a patient is clearly not a candidate for any treatment, all patients should undergo a chest computed tomography (CT) scan appropriately modified for the purposes of lung cancer assessment. The lung cancer CT scan includes the chest and the upper abdomen such that the liver and the adrenal glands are visualized in their entirety. The administration of intravenous contrast facilitates better characterization of hilar and mediastinal lymph nodes by differentiating them from vascular structures and also helps to better characterize hepatic and adrenal lesions. CT is most useful in delineating the key features of the primary tumor (size, presence of satellite nodules, associated atelectasis and infection, and invasion of adjacent structures); these features are essential in establishing the clinical T stage.
Regional lymph node involvement can also be evaluated on chest CT. Lymph nodes are deemed to be enlarged when they measure greater than 1 cm in short-axis diameter on transverse images. Likelihood of metastatic involvement increases with the degree with mediastinal enlargement: 62% of lymph nodes measuring 10 to 15 mm are malignant versus 90% of nodes measuring >15 mm.36 However, in most patients, lymph node staging cannot be accurately undertaken based upon CT findings alone. In a meta-analysis of 35 studies (5111 patients, 28% of whom had mediastinal metastases), the pooled sensitivity of CT scanning for mediastinal nodal metastases was found to be 51% and pooled specificity 86%.37 About 20% of nodes deemed to be benign on chest CT were actually malignant and 40% of nodes deemed abnormal on chest CT were actually benign. On occasion, there are CT findings that demonstrate mediastinal tumor invasion convincingly enough that further staging is unnecessary. These findings include extensive infiltration of mediastinal structures such that discrete anatomic landmarks are lost, matting of nodal tissue such that boundaries become indistinct, and encircling of mediastinal structures by infiltrating tumor. In the absence of these findings, further studies need to be undertaken to adequately stage the mediastinum.
Chest CT can also often demonstrate findings that may be suggestive of metastatic disease. CT may better delineate pleural disease; the majority of these effusions are malignant. In addition, CT may reveal pericardial effusion, pleural nodularity, or nodules in the contralateral lung, all of which may indicate metastatic disease. Bony involvement of the thoracic cage may be seen. Adrenal lesions can be identified in up to 10% of patients, however, many of these are ultimately found to be benign.38
Positron emission tomography (PET) with 18-fluoro-2-deoxyglucose (FDG) has assumed an increasingly prominent role in the initial noninvasive evaluation of the patient with suspected lung cancer.39 Metabolically active tissues take up FDG, a glucose analog, avidly and appear bright on PET scan. Strongly hypermetabolic activity raises suspicion of malignancy. However, hypermetabolic activity can also be seen in inflammatory processes related to infections or recent surgery, raising the specter of false positivity. False negatives can occur with low-grade malignancies or small foci of malignancy. Lesions less than 0.8 to 1.0 cm may be too small for accurate assessment. High-level physiologic uptake within the brain, heart, gastrointestinal, and genitourinary tracts may compromise precise evaluation of tumor invasion in these areas. In addition, the presence of hyperglycemia can interfere with the uptake of FDG and thus increase the likelihood of false-negative examinations. Integrated PET/CT allows more detailed evaluation of abnormal tissues and surrounding structures and it is more helpful than full-body PET, which has limited anatomic resolution, to elucidate specific tumor characteristics as well as surrounding structures.
The PET scan is more sensitive and specific than CT in regional lymph node assessment with a pooled sensitivity of 74% and specificity of 85% in the identification of mediastinal lymph node metastases.37 In patients with enlarged mediastinal nodes, the sensitivity has been estimated to be as high as 100% with a specificity of 78%.40 Thus the false-positive rate ranges between 13% and 25% in patients with nodal enlargement. In those with normal-sized nodes on CT, PET sensitivity is 82% but specificity is higher at 93%.41 Approximately 20% of patients with normal mediastinal nodes on CT and PET will actually have malignant disease within the mediastinum.
PET scanning can disclose unsuspected metastatic disease (M1) in 6% to 37% of cases, depending on clinical pretest probability. Rates are lower in earlier-stage disease: 1% to 8% of patients with clinical stage I disease and 7% to 18% in patients with clinical stage II disease.37 Liver metastases, adrenal metastases, and bone metastases can be diagnosed with good accuracy. A recent study of PET-CT in the evaluation of adrenal lesions detected in patients with lung cancers reported an overall sensitivity and specificity of 97% and 94%, respectively.42 Two systematic reviews suggest that PET scanning has higher diagnostic value than bone scintigraphy for the detection of bone metastases from lung cancer, with >90% sensitivity and specificity for PET-CT.40,43
PET has been the most helpful in decreasing the frequency of understaging in patients at risk for advanced lung cancer. Clinical trials have suggested that addition of PET imaging to the evaluation results in a reduction of the rates of noncurative surgery for NSCLC from 40% down to 20%.44,45 The frequency of understaging disease decreases from 30% to 15%. The beneficial impact of PET diminishes in patient with early-stage disease. In patients with clinical stage I disease, PET correctly detects N2, N3, or M1 disease in 7% to 10%.46 However, this comes at a cost of increased false-positive rate of up to 14%. PET should not be used alone to confirm advanced stage disease as patients may be erroneously directed away from curative therapies.
MRI scanning is the test of choice when assessing for intracranial metastatic disease. It is more sensitive than CT scan of the brain.47 Staging brain MRI should be considered in patients who present with locally advanced or advanced NSCLC on chest CT or PET scanning, patients with neurologic signs or symptoms, patients presenting with nonspecific constitutional symptoms of fevers, weight loss, anorexia, and weakness and in patients with SCLC. MRI scanning is also often the test of choice for focused examination for extent of chest wall invasion, diaphragmatic involvement, superior sulcus tumors, brachial plexus invasion, or invasion of the spine (T4 disease).
Any extrathoracic lesion detected on imaging studies in a patient suspected of having lung cancer should be biopsied first, if feasible. This will accomplish not only tissue diagnosis but also confirmation of advanced disease in both SCLC and NSCLC.
ESTABLISHING THE TISSUE DIAGNOSIS
Methods employed in establishing a tissue diagnosis SCLC and NSCLC are discussed below.
 SCLC
SCLC
Specific imaging characteristics on chest CT can be indicative of SCLC. In particular, the findings of massive lymphadenopathy, direct mediastinal invasion, or hilar masses can suggest SCLC. The suspicion should be raised if clinical evaluation is worrisome for SIADH, ectopic ACTH production, or neurologic paraneoplastic syndromes. If SCLC is suspected, then tissue diagnosis should be established utilizing the least invasive test feasible.
In 1989, the International Association for the Study of Lung Cancer modified the Veterans Administration Lung Study Group (VALSG) two-stage classification of SCLC; this modified classification system is currently the widely accepted clinical staging system for SCLC.48 Patients with limited stage disease have disease confined to one hemithorax, the mediastinum, and ipsilateral supraclavicular lymph nodes. In other words, limited stage defines a primary tumor and regional nodes that can be incorporated into in a reasonably safe radiation port. Patients with extensive stage disease have malignant pleural and pericardial effusions, contralateral hilar or supraclavicular nodes, and/or distant metastases. Given the high likelihood of extensive disease at the time of presentation, all patients should undergo a full staging evaluation that traditionally included a contrast-enhanced CT of the chest and abdomen, bone scan, and MRI or contrast-enhanced CT scan of the brain. MRI scanning, the preferred imaging modality, will detect brain metastasis in 10% to 15% of asymptomatic patients with SCLC at the time of diagnosis, and in 12% of patients with otherwise limited stage SCLC.34,49 Routine bone marrow aspiration is not indicated. The use of PET scan in the initial stating of SCLC has been evaluated in several small studies.50–54 Because SCLC is a highly metabolic malignancy, PET scan is 100% sensitive for the detection of primary tumor. In addition, PET is superior to standard imaging in both sensitivity and specificity for detection of non-CNS metastatic foci. In patients with radiographic evidence of extensive stage SCLC, tissue diagnosis, and stage confirmation should be accomplished by biopsy of a metastatic focus if feasible.
 NSCLC
NSCLC
In the case of NSCLC, the primary goals in tissue sampling selection are more complicated. These goals are to (1) obtain a tissue specimen adequate for histopathology and genetic analysis and (2) establish the clinical stage of disease in the least invasive manner feasible. If imaging tests suggest a site of distant metastatic disease, then that site should be sampled first, if accessible.
Sputum cytology is the least invasive way of achieving a histologic diagnosis. However, yield is clearly center dependent and requires a highly trained respiratory cytologist to process and analyze specimens appropriately. Sensitivity of sputum cytology has been reported to be as low as 42% and as high as 97%.55 Yield increases with number of specimens submitted: 68% for one specimen, 78% for two, and 86% when three or more specimens are submitted.56 Diagnostic yield is higher in patients with central tumors, tumors >2.4 cm, squamous cell histopathology, and those with hemoptysis.55 Diagnostic yield is lowest in patients with peripheral lesions.
Thoracentesis is indicated when a pleural effusion is present as a significant proportion of pleural effusions seen in patients with lung cancer are malignant. Positive pleural fluid cytology not only establishes the diagnosis but also establishes stage IV disease in NSCLC and extensive stage SCLC. Thoracentesis is best accomplished utilizing bedside ultrasound guidance, as this appears to improve the safety and yield of the procedure. Furthermore, ultrasound imaging can reveal findings that raise the suspicion for malignant disease, including pleural thickening and nodularity. A volume of at least 50 mL should be sent; diagnostic yield does not appear to increase with larger volume of fluid analyzed.57 If the initial thoracentesis is negative, a second thoracentesis should be performed. A second thoracentesis has a diagnostic yield of 27% when the first is negative. Sensitivity of pleural fluid cytology can be as high as 72% when at least two separate specimens are sent.55
When pleural disease is present and thoracentesis is negative, additional diagnostic strategies include blind pleural biopsy, percutaneous image-guided biopsy, and thoracoscopic biopsy. Non-image guided closed pleural biopsy using an Abrams needle probably no longer has a role as it increases diagnostic yield over thoracentesis by only 7% to 27%.55 Image-guided pleural biopsy has clear advantages in diagnostic yield, particularly when there are abnormal findings, with a sensitivity of 84%.55 Thoracoscopy has a reported sensitivity of 80% to 99% in the diagnosis of malignant pleural disease.55 However, when there is CT evidence of pleural thickening or nodules, the diagnostic yield of image-guided percutaneous biopsy is similar to thoracoscopy. Thus, medical or surgical thoracoscopy may be reserved for patients with pleural fluid but without a discrete target for CT-guided pleural biopsy.58
HISTOLOGIC SAMPLING OF THE PRIMARY TUMOR
There are numerous procedures presently utilized to sample the primary tumor with varying diagnostic yields depending on tumor characteristics and adjunct technologies (Table 112-5). Bronchoscopy enables sampling of the primary tumor as well as the mediastinum for regional lymph node staging. Overall sensitivity of endobronchial forceps biopsy of directly visualized central tumor is 74% with yield increasing with the number of biopsy passes.55 It is recommended that at least three biopsies be taken. The yield of endobronchial washes and brushes is lower (sensitivity 47% and 61%, respectively) but may have additive benefit.55 Endobronchial needle aspiration further increases yield as forceps biopsies of endobronchial lesions can sometimes sample areas of necrosis and may result in nondiagnostic biopsies.59–61 When all techniques are utilized, reported yield of bronchoscopic sampling of endobronchial lesions can be as high as 91% to 96%. Bronchoscopic visualization of endobronchial disease is also the best way to estimate the extent of proximal tumor invasion, specifically if tumor invasion occurs proximal to a lobar bronchus (T2b lesion) or invades the mainstem bronchus to within 2 cm of the carina (T3 disease).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree