Chapter 81 Clinical Application of New Antiarrhythmic Drugs for Atrial Fibrillation
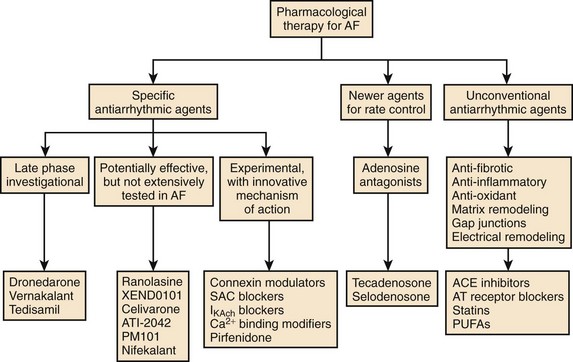
Atrial fibrillation (AF) is the most common sustained arrhythmia requiring medical care. Oral antiarrhythmic drugs are effective in controlling AF in 50% to 65% of cases but have limitations, including subjective adverse effects, ventricular proarrhythmia, and end-organ toxicity.1 In addition, therapeutic options available to the majority of patients with AF associated with significant structural heart disease are limited. Therefore, more effective and safer antiarrhythmic drugs for the termination and prevention of AF are needed (Figure 81-1).2 Although many new antiarrhythmic drugs are at various degrees of development, this chapter will limit discussion to a recently released antiarrhythmic, dronedarone, a commercially released anti-anginal medication with antiarrhythmic properties; vernakalant; several drugs furthest along in development, such as celivarone, budiodarone, and ranolazine.
Dronedarone (Multaq)
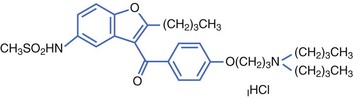
Dronedarone is a non-iodinated benzofurane derivative, structurally related to amiodarone, developed by Sanofi-Aventis. The addition of a methyl-sulfonamide grouping made the drug less lipophilic with a shorter half-life than that of amiodarone (Figure 81-2).
Electrophysiology
In vitro, dronedarone blocks IKr, IKs, Ito, INa, and ICa-L.3 Dronedarone prolongs the action potential duration in the atria and ventricles with no significant reverse-use dependence. In vivo, dronedarone has been shown to actively block the following channels: INa, IKr, IKs, IKACh, and ICa-L and inhibit α-adrenoceptors and β-adrenoceptors. Although its effect is similar to that of amiodarone, the magnitude of the effect is different. For example, dronedarone is 10 times more effective in blocking INa, and 100 times more effective in blocking IKACh, compared with amiodarone. In addition, blockade of isoprenaline β2-adrenoceptor–mediated decrease in blood pressure is more pronounced with dronedarone than with amiodarone. Other amiodarone-like electrophysiological effects include alpha, beta, and muscarinic blocking effects.3 Dronedarone slows sinus rate, prolongs atrioventricular (AV) nodal refractory periods, and slows AV nodal conduction. Dronedarone increases the Q-T interval but has a low propensity to cause torsades de pointes, since dronedarone reduces the transmural dispersion of ventricular refractoriness and protects from class III antiarrhythmic-induced early after depolarizations.3
Dronedarone exhibits significant antiarrhythmic properties in the ventricle and is effective against arrhythmias arising as a result of myocardial ischemia; in murine studies, it has also been shown to be effective against arrhythmias arising as a result of sudden reperfusion of the ischemic myocardium. In ischemic porcine hearts, dronedarone appears to be more potent than amiodarone in suppressing ventricular arrhythmias.3
Pharmacokinetics and Metabolism
Similar to amiodarone, over a two-fold increase is seen in the serum concentration of dronedarone when taken with food. With twice-daily dosing, the drug reaches steady-state levels in 4 to 7 days. The elimination half-life of dronedarone ranges from 13 to 30 hours.3 Dronedarone is a substrate for and an inhibitor of CYP3A4. Therefore, dronedarone should not be used concomitantly with potent CYP3A4 inhibitors such as ketokonazole or macrolide antibiotics. Dronedarone has similar amiodarone-like drug interactions with simvastatin and digoxin, but, importantly, no significant dronedarone interaction with warfarin occurs. Dronedarone increases the tubular secretion of creatinine by about 10%, with no effect on the glomerular filtration rate.
Clinical Efficacy
Several clinical trials have assessed the efficacy of dronedarone in suppressing AF in humans. The Dronedarone Atrial Fibrillation study after Electrical Cardioversion (DAFNE) was the first prospective randomized trial evaluating the efficacy and safety of dronedarone.4 In this dose-ranging study, placebo or dronedarone 400 mg, 600 mg, or 800 mg twice daily was randomly given to 199 patients with AF longer than 3 days but less than 365 days. Patients were then followed up for 6 months to measure the primary endpoint of time to AF recurrence. In comparison with placebo, dronedarone 400 mg twice daily significantly prolonged the time to recurrence of AF (median time 60 days in the dronedarone group versus 5.3 days in the placebo group, P < .001; relative risk reduction, 55%; confidence interval [CI], 28% to 72%). At doses higher than 400 mg twice daily, no efficacy in preventing the recurrence of AF was noted; an adverse effect—a dose-response curve of gastrointestinal side effects (diarrhea, nausea) requiring drug discontinuation—was, however, noted. The DAFNE demonstrated that dronedarone, at a dose of 400 mg twice daily, was safe and effective in preventing AF recurrences following conversion of persistent AF but was not very effective in the medical conversion of persistent AF.
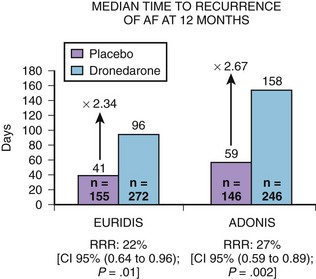
Two pivotal phase III trials assessed the efficacy of dronedarone in the maintenance of sinus rhythm in patients with AF or atrial flutter: (1) The Australian-American-African Trial with Dronedarone in Atrial Fibrillation or Flutter for the Maintenance of Sinus Rhythm (ADONIS) and (2) the European Trial in Atrial Fibrillation or Flutter in Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm (EURIDIS).5 These blinded, placebo-controlled trials randomized patients in a 2 : 1 ratio of either placebo or dronedarone 400 mg twice daily (Figure 81-3). Patients had a history of AF or atrial flutter in the previous 3 months and had to be in sinus rhythm for at least 1 hour before randomization. In the two trials, 1237 patients were randomized to either dronedarone or placebo (612 in the EURIDIS trial and 625 in the ADONIS trial). In the EURIDIS trial, dronedarone prolonged the median time to recurrence of AF or atrial flutter from 41 days in the placebo group to 96 days (relative risk [RR], 0.784; P = .0138). In ADONIS, the median time to recurrence increased from 59 days in the placebo group to 158 days in the dronedarone group (RR, 0.725; P < .0017). Dronedarone also significantly reduced symptomatic AF recurrences and significantly slowed the ventricular response rate by about 12 to 15 beats/min compared with placebo (P < .001) in those who had AF recurrence.
In the Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation (ERATO) trial, in addition to other AV node–blocking agents, dronedarone had therapeutic use in further slowing ventricular response during permanent AF or atrial flutter as assessed by Holter monitoring and stress testing.6
As part of the European regulatory filing, the active control Randomized, Double-Blind Trial to Evaluate the Efficacy and Safety of Dronedarone (400 mg bid) Versus Amiodarone (600 mg qd for 28 Days, then 200 mg qd Thereafter) for at Least 6 Months for the Maintenance of Sinus Rhythm in Patients with AF (DIONYSOS), which compared dronedarone with amiodarone, was performed in 504 patients with persistent AF.7 The primary endpoint of this trial was a composite of electrocardiographically documented AF recurrence or premature discontinuation of drug for lack of efficacy or adverse events. The trial had a short follow-up period (mean, 7 months) but was able to show that amiodarone was more effective in suppressing the recurrence of AF but tended to having more adverse events, including thyroid abnormalities and bleeding secondary to amiodarone-warfarin interaction problems.
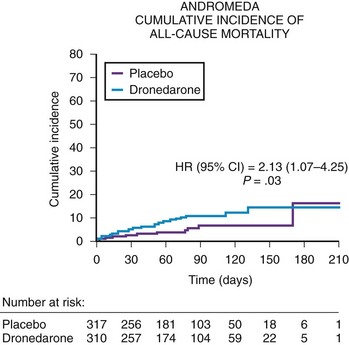
The safety of dronedarone in moderate to severe congestive heart failure (CHF) was assessed in the Antiarrhythmic Trial with Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease (ANDROMEDA) trial.8 The ANDROMEDA trial was a double-blind, placebo-controlled study evaluating dronedarone (400 mg twice daily) in high-risk patients with CHF and ventricular dysfunction (Figure 81-4). The primary endpoint of the ANDROMEDA trial was the composite endpoint of all-cause mortality or hospitalization for heart failure. The ANDROMEDA trial focused on patients at the highest possible risk of major cardiovascular events and enrolled recently hospitalized patients having current symptomatic New York Heart Association (NHYA) class II to IV heart failure with at least one decompensation of heart failure (class III to IV) in the last month and a wall motion index (WMI) by echocardiography of 1.2 or less, which correlates to a left ventricular ejection fraction of 35% or lower. Except for the Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) trials with dofetilide, patients hospitalized for decompensated heart failure generally had not been enrolled in survival trials to evaluate the safety of antiarrhythmic drugs.9 After enrolling 627 of the 1000 planned patients, the trial was stopped by the Data and Safety Monitoring Board because of the excess risk of death (risk ratio, 2.13) in patients treated with dronedarone. In a review of the cause of deaths in this trial, the majority of deaths were secondary to nonsudden death or worsening of heart failure in the dronedarone arm. The primary composite endpoint of mortality and cardiovascular hospitalization trended adversely for dronedarone but was not statistically different (hazard ratio [HR], 1.38; CI, 0.92 to 2.09). The most likely explanation is that the ANDROMEDA trial finding is a true finding that can be explained by the deleterious effect of dronedarone in NYHA class III to IV patients with left ventricular ejection fractions of less than 35% who also had a recent hospitalization for decompensated heart failure. Similar concern has been raised by the termination of the recent Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS) trial.
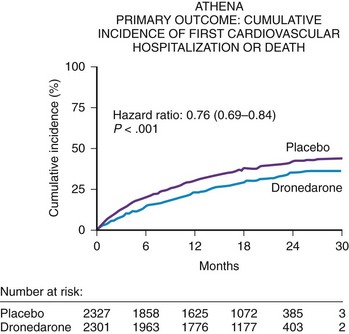
The A Placebo-Controlled, Double-Blind Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter (ATHENA) trial was conducted to focus on patients at risk of AF recurrence who may or may not have had heart failure but who would not have been randomized in the ANDROMEDA trial.10 Thus, key exclusion criteria for the ATHENA trial were pulmonary edema within 12 hours, cardiogenic shock requiring intravenous pressors, mechanical ventilation or class IV heart failure within 4 weeks, or all. The ATHENA trial was also performed for several other regulatory reasons: (1) to verify the post hoc result from the ADONIS and EURIDIS trials that dronedarone prospectively could reduce the composite endpoint of cardiovascular hospitalizations or death; (2) to create a large database to show that dronedarone would be safe in a large number of patients with structural heart disease; and, (3) to define a point estimate to show that this drug could be used safely in high-risk patients with AF or atrial flutter excluding the ANDROMEDA trial population.
The ATHENA trial demonstrated a statistical reduction in the composite endpoint of all-cause mortality or cardiovascular hospitalization in the dronedarone group (HR, 0.76; CI, 0.69 to 0.84; P < .001)] (Figure 81-5). Although dronedarone reduced cardiovascular hospitalization rates (HR, 0.75; CI, 0.67 to 0.82), no effect was seen on reducing hospitalizations for noncardiovascular reasons (HR, 0.98; CI, 0.87 to 1.11). The decrease in re-admission for cardiovascular hospitalization was mainly determined by the suppression of AF and other supraventricular disorders (HR, 0.62; CI, 0.53 to 0.71). All-cause mortality trended favorably in the dronedarone group with an HR of 0.84 (CI, 0.66 to 1.08); cardiovascular death relative risk was 0.70 (CI, 0.51 to 0.96). A retrospective analysis of the ATHENA trial reported that dronedarone reduced the incidence of stroke by 34% (HR, 0.66; CI, 0.46 to 0.96).11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree