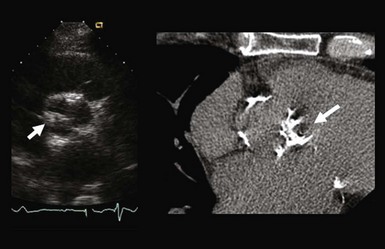
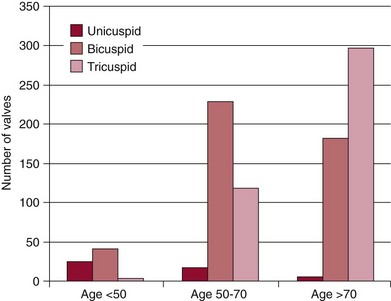
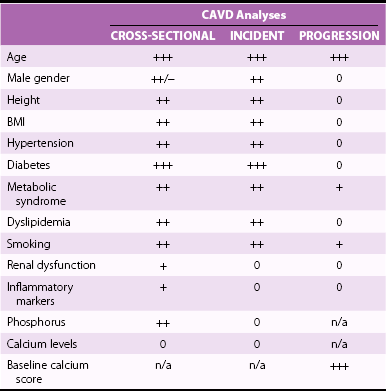
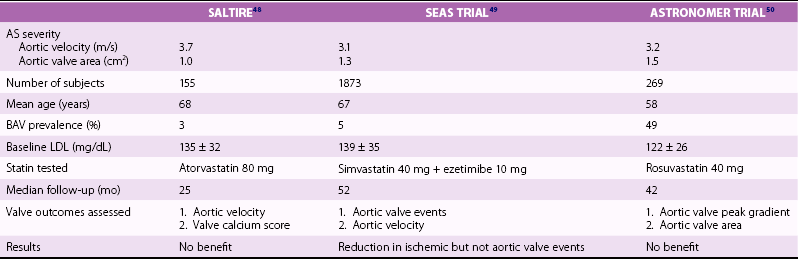
Chapter 4 Calcific aortic valve disease, defined by aortic valve leaflet thickening and calcification, is a common feature of aging, being present in nearly 25% of individuals older than 65 years, and in more than 40% of individuals more than 75 years of age. 1 Because of its high prevalence among the elderly, CAVD was initially thought to be a degenerative process due to wear and tear of fragile leaflets. However, contemporary research has demonstrated CAVD to be an active biologic process marked by lipoprotein deposition, inflammatory cell infiltration, renin-angiotensin system activation, and calcium deposition (further discussed in Chapter 3). To date, no lifestyle modifications or medical therapies have been shown to slow CAVD initiation or progression, and the mainstays of therapy remain careful observation and timely procedural intervention with surgical or transcutaneous aortic valve replacement (AVR) at the onset of valve-related symptoms.2,3 CAVD is one of the most common indications for cardiac surgery, with approximately 75,000 AVR procedures performed annually in the United States. In contrast, CT is well validated as a modality for assessing CAVD presence and severity, particularly in the earlier disease stage.4–6 Although its clinical role is limited because CT cannot assess the hemodynamic sequelae of valve calcification, it remains a powerful research tool. With use of the Agatston method, which estimates calcium volume and density from voxel intensity, 7 CT allows quantitative assessment of calcium burden in all stages of disease. Moreover, CT is relatively inexpensive and easily acquired and has been incorporated into many large epidemiologic studies. Modern, high-resolution CT technology may also permit assessment of valve morphology. Figure 4-1 shows the appearance of aortic stenosis (AS) on both echocardiography and CT. Although valvular interventions are occasionally necessary in childhood, adults with BAV are at risk for early valve calcification, clinically significant AS, and surgical valve replacement. The importance of BAV in CAVD pathogenesis was demonstrated by rigorous, pathologic analyses of explanted aortic valves ( Figure 4-2).8,9 In a series of 932 patients undergoing AVR, Roberts et al 9 estimated that nearly 50% had a BAV disease and that patients with BAV underwent AVR approximately 2 decades earlier than those with normal trileaflet valves. BAV was present in about 62% of patients undergoing AVR who were aged 50 to 70 years, but in only 37% of those older than 70 years. An earlier, small case series of 43 patients found a 42% prevalence of BAV among surgically resected aortic valves. 10 FIGURE 4-2 Valve morphology among subjects undergoing aortic valve replacement, classified by age. (Data from Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005;111:920–5.) Early family studies suggested that BAV disease may have a genetic basis. Initial reports of familial clustering prompted detailed family screening studies, which showed that approximately 9% of first-degree relatives of patients with BAV also had BAV morphology.11,12 From these studies, it was estimated that the “heritability factor” for BAV disease was approximately 89%, suggesting a strong genetic basis with low phenotypic penetrance. Subsequent linkage analyses identified possible loci at 18q, 5q, and 13q, but exact genes were not identified. 13 In a landmark study, Garg et al 14 studied a large family that had both highly penetrant BAV disease and other congenital heart abnormalities. Using linkage-guided gene sequencing, the researchers identified a stop mutation in the NOTCH1 gene. This result was further validated in an independent study of a BAV disease family, in whom a NOTCH1 frameshift mutation was then identified. NOTCH1 is highly expressed in aortic valve development and normally suppresses Runx2, a transcription factor that regulates osteoblastogenesis. Although mutations in NOTCH1 are extremely rare in the population, explaining only a small portion of BAV and CAVD, this proof-of-concept discovery demonstrates that genetic abnormalities may underlie the BAV phenotype and early valve calcification. This limitation aside, observational and epidemiologic studies have provided important insights into the risk factors for CAVD in varied stages. The earliest of these studies focused on cross-sectional risk associations in subjects with end-stage CAVD, whereas later studies have utilized CT to identify risk associations in earlier-stage disease. In general, traditional cardiovascular risk factors have shown strong associations with the presence of CAVD and incident CAVD but weak associations with CAVD progression (summarized in Table 4-1). The following discussion summarizes the major findings. TABLE 4-1 General Summary of Strength of Associations Seen in Observational and Epidemiologic Studies of Clinical Risk Factors and Calcific Aortic Valve Disease CAVD primarily affects the elderly, and the prevalence of CAVD increases with advancing age. In the Cardiovascular Health Study, CAVD prevalence was 21% in subjects 65 to 74 years old, 38% in those 75 to 84 years old, and 52% in those 85 years or older. 1 Longitudinal analyses from the Multi-ethnic Study of Atherosclerosis (MESA) show not only that this increasing prevalence is due to accumulation of cases but also that the rate of new (“incident”) CAVD also rises substantially with age. For example, the estimated CAVD incidence rate is 0.6% per year in subjects 50 to 54 years old and 3.5% per year in those 70 to 79 years old ( Figure 4-3). 15 FIGURE 4-3 Incidence rate of new aortic valve calcium (AVC) by age. Age also has been shown to be an important modifier of the association between traditional cardiovascular risk factors and CAVD. Owens et al 16 demonstrated that age significantly modified the low-density lipoprotein cholesterol (LDL)–associated risk of CAVD, with LDL being a risk factor in younger but not older individuals within the MESA population. Similar findings were seen in a cohort of patients with AS undergoing AVR, in which elderly subjects had less atherogenic lipid profiles in general. 17 In a later study, a significant interaction between age and the metabolic syndrome was shown in patients with severe AS, such that the effect of metabolic syndrome was stronger in younger patients. 18 Overall, these findings suggest weaker influences of traditional cardiovascular risk factors in older subjects, which in turn has two implications. First, treatment of cardiovascular risk factors to prevent CAVD may be most beneficial in younger patients; and second, other mechanisms may be important in older individuals, in whom the rate of incident CAVD is the highest. On multivariate analyses, most studies have identified male gender to be associated with CAVD in both early-stage and late-stage disease. In the MESA, which utilized CT to identify early subclinical disease, male gender conferred a 1.87-fold (95% confidence interval (CI) 1.31-2.69) higher odds of valve calcium after adjustment for traditional cardiovascular risk factors. 19 One study, however, found a strong independent association between aortic sclerosis identified by echocardiography and female gender. 20 Few studies have examined the relationship between CAVD and race/ethnicity, and the majority of clinical data surrounding CAVD is derived from Caucasian populations. One study examining interracial differences in BAVs demonstrated a significantly higher prevalence of BAVs among Caucasians than among African-Americans with a single-center cohort (1.1% versus 0.2%; P = 0.001). 21 The MESA used targeted oversampling of ethnic minority groups in its recruitment in order to enhance statistical power. In the MESA population, the baseline prevalence among the race/ethnic groups was 14% among white, 7% among Chinese, 11% among black, and 12% among Hispanic participants. 16 However, after baseline cardiovascular risk factors were accounted for, there was no significant difference among these race/ethnic groups in either cross-sectional or incident analyses.16,19 However, on pathologic analysis of explanted stenotic aortic valves, African-Americans showed greater likelihood of heterotopic ossification (bone formation). Whether this is due to delayed surgical intervention from socioeconomic factors or an inherent tendency for bone formation is unclear. 22 Studies have accounted for body size in various manners, including assessment of height, weight, body mass index (BMI), and waist circumference. The relationship between height and CAVD appears complex. Within the Cardiovascular Health Study (CHS), greater height was associated with prevalent aortic sclerosis, 1 but short stature was associated with progression to AS. 23 Height may influence aortic pulse wave dynamics, thus altering shear forces across the aortic valve. Multiple case-control, population, and prospective studies have shown an association between hypertension and CAVD. Linefsky et al 24 demonstrated that higher hypertension categories were more strongly associated with prevalent disease in the MESA population. Additionally, Iwata et al 25 provide compelling data associating 24-hour ambulatory blood pressure with the cross-sectional prevalence of aortic sclerosis. 25 After adjustment for cardiovascular risk factors, 24-hour awake/asleep mean diastolic pressures—but not systolic pressures—were associated with aortic sclerosis. This finding is intriguing, because diastolic flow across the aortic side of the valve leaflets and into the coronary arteries creates shear forces that may contribute to disease initiation and progression. However, this finding has not been validated on a prospective basis, and data supporting a role for hypertension in disease progression are currently lacking. Insulin resistance, diabetes, and the metabolic syndrome form a spectrum of metabolic abnormalities that have been shown to associate strongly with coronary atherosclerosis. The Homeostatic Model of Assessment—Insulin Resistance (HOMA-IR) has been associated with incident CAVD in prospective analysis of the MESA cohort, but this association was not independent of other cardiovascular risks. 26 Similarly, overt diabetes has not been a consistent predictor of either cross-sectional CAVD or incident CAVD, nor a predictor of CAVD progression. Case-control studies have shown mixed results,20,27,28 and diabetes was found to be associated with cross-sectional CAVD and incident CAVD in the MESA population.29,30 However, this result was not found in either the Cardiovascular Heart Study or the Framingham Heart Study.1,23,31 Interestingly, patients with diabetes had a lower rate of heterotopic ossification on histopathologic evaluation of explanted, severely calcified aortic valves of patients undergoing valve replacement. 22 Metabolic syndrome is a constellation of clinical conditions that are often coexistent, including dysglycemia and insulin resistance, high blood pressure, dyslipidemia, central obesity, and microalbuminuria.32–34 Patients with metabolic syndrome have a higher prevalence of CAVD 30 and are more likely to have incident CAVD ( Figure 4-4). 29 Additionally, metabolic syndrome appears to influence the rate of hemodynamic progression of late-stage CAVD measured either by transvalvular velocity 18 or aortic valve area. 35 The metabolic syndrome has also been associated with faster deterioration of bioprosthetic aortic valves.36,37 Although metabolic syndrome is one of the few clinical factors that have been shown to be associated with CAVD progression, the mechanisms underlying this association are unclear. FIGURE 4-4 Rates of incident aortic valve calcification (AVC) in relationship to diabetes (DM) and metabolic syndrome (MetS). Multiple observational studies have demonstrated significant associations between atherogenic dyslipidemia, including both total cholesterol and LDL concentrations, and both prevalent and incident CAVD.1,19,20,23,31,38,39 However, the risk conferred by dyslipidemias appears to be clinically modest. Additionally, at least one study found an association between lipoprotein(a) [Lp(a)] concentrations and the presence of CAVD, 1 and Lp(a) has been shown to colocalize to regions of calcification. 40 These observational studies were bolstered by hyperlipidemic animal models,41,42 in vitro studies,43,44 and retrospective clinical analyses,45–47 suggesting a beneficial effect of hydroxymethyl glutaryl–coenzyme A (HMG-CoA) reductase inhibition with statin therapy in slowing the progression of CAVD. Together, these findings, for a time, fostered a paradigm of CAVD as an atherosclerosis-like process. Several randomized clinical trials were launched to test the hypothesis that statin therapy would slow progression of established CAVD ( Table 4-2). The first of these, the Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE), 48 tested whether 80 mg of atorvastatin daily would slow CAVD progression (as measured by aortic valve jet velocity and aortic valve calcium score) in 156 patients with established AS (mean jet velocity 3.4 m/s). Over a median follow-up of 25 months, the rates of AS progression did not differ between the treated and untreated groups. A second, much larger trial, the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) study, tested whether simvastatin 40 mg plus ezetimibe 10 mg daily would slow CAVD progression 49 in 1873 patients with mild-to-moderate AS; after a median follow-up of 52 months, the between-group rate of aortic valve replacement did not differ (28.3% vs. 29.9%; P = 0.97). A third trial, the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER), 50 randomly assigned 269 subjects with asymptomatic AS to receive either rosuvastatin 40 mg daily or placebo. Although this population was notable for being younger (mean age 58 years) and having a high prevalence of BAV morphology (49%), there was no significant difference between the groups in the transvalvular gradients or aortic valve areas at a mean follow-up of 3.5 years. TABLE 4-2 Summary of Randomized Control Trials Testing Statin Therapy for Slowing the Progression of Calcific Aortic Valve Disease
Clinical and Genetic Risk Factors for Calcific Valve Disease
Methods of Detection
Bicuspid Aortic Valves
Clinical Risk Factors
Older Age
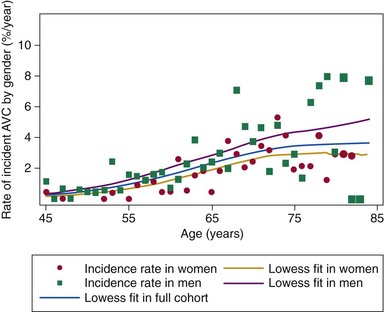
Data over a median follow-up of 2.7 years are shown for the 5142 participants in the Multi-Ethnic Study of Atherosclerosis who were free of baseline AVC. (The size of the scatter points is weighted for the number at risk in each age category.) A marked increase in the AVC incidence rate is seen with advancing age. (From Owens DS, Katz R, Takasu J, et al. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis [MESA]. Am J Cardiol 2010;105:701–8.)
Male Gender
Race/Ethnicity
Anthropometry
Hypertension
Dysglycemia and the Metabolic Syndrome
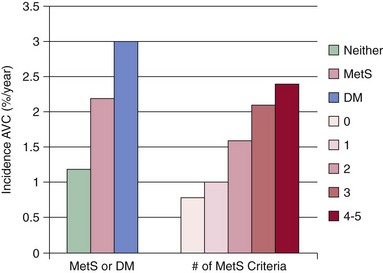
Data are shown for the 5723 participants in the Multi-ethnic Study of Atherosclerosis, according to (left) the presence of metabolic syndrome (by criteria of the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [ATP-III]) or diabetes and (right) the number of metabolic syndrome criteria present. (Adapted from Katz R, Budoff MJ, Takasu J, et al. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes 2009;58:813–9.)
Dyslipidemia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Clinical and Genetic Risk Factors for Calcific Valve Disease
Only gold members can continue reading. Log In or Register to continue