Chronic Beryllium Disease and Hard-Metal Lung Diseases
CHRONIC BERYLLIUM DISEASE
Beryllium is the lightest metal and has an atomic number 4. Gem stones, such as aquamarine, emerald, and beryl contain beryllium and have been recognized since ancient times. But beryllium, as an element, was first discovered in 1798 by the French chemist, Vauquelin and reduced to its metallic form; subsequently, it was named beryllium in 1828 by the German metallurgist, Wohler. Beryllium became a commercial product when it was used as an alloy first with aluminum and later with copper, nickel, and cobalt after World War I. The industry grew in the 1930s due to the increased use of beryllium–copper products during World War II and the use of beryllium oxide in the refractory and fluorescent lamp industries. During and after World War II, beryllium was used in the nuclear industry because of its ability to function as a neutron multiplier. Beryllium was used for both civilian nuclear reactors and for military weapons.
With an increased industrial need for beryllium, acute chemical pneumonitis was first described by Weber and Engelhardt1 in Germany in 1933 and in the United States by van Ordstrand et al. in 1943.2 This condition was usually limited to the upper respiratory tract, though it could extend to the bronchi, bronchioli, and alveoli if there was sufficient exposure. This condition peaked in the 1940s and will not only be seen if there are plant explosions or other serious lapses in procedures.3 The last reported possible case in the United States occurred in the early 1980s.
A second pulmonary complication of beryllium exposure was first described by Hardy and Tabershaw4 in 1946. This disease differed from the acute chemical pneumonitis because of the delayed onset, granulomatous response, and chronic course. Now known as chronic beryllium disease (CBD), this condition is a hypersensitivity reaction to beryllium and is the major hazard facing beryllium workers today.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
CBD is primarily a pulmonary granulomatous disorder. Although involvement of other organ systems has been reported (e.g., lymph node, skin, and liver), the lungs are the principal organ affected and account for the morbidity and mortality of this disease.5,6 In the early stages, CBD may be asymptomatic. A positive blood proliferative response to beryllium (evidence for beryllium hypersensitivity) may be the earliest sign of CBD.7,8 Radiologic changes can also be detected on routine chest radiographs. Symptomatic disease usually begins with nonspecific respiratory complaints, such as dyspnea and cough. Early in the disease process, routine chest radiography may not be helpful. Pulmonary function testing early in the disease may be normal or have an isolated abnormality of the diffusing capacity (DLCO).9 As the disease progresses, symptoms become more characteristic for chronic interstitial lung disease (ILD) with a nonproductive cough, substernal burning pain, and progressive exertional dyspnea. At this stage dry bibasilar crackles are observed on physical examination. A rare patient may have asthmatic-type complaints and physical findings. With advanced disease progressive weakness, easy fatigability, dyspnea at rest, anorexia, and weight loss may occur and acrocyanosis and clubbing may be observed. As cor pulmonale develops, peripheral edema, hepatomegaly, and distended neck veins are seen.10 Fever is unusual but can be seen. Hypercalcemia and nephrocalcinosis, hyperuricemia, joint pains, and severe cachexia have been described. Severe liver involvement has not been seen, but liver granulomas with mild elevation of the liver function tests occur. Skin involvement may occur in 10% to 30% of cases and frequently involves small granulomatous nodules on the hands, arms, and chest.5
 RADIOGRAPHY
RADIOGRAPHY
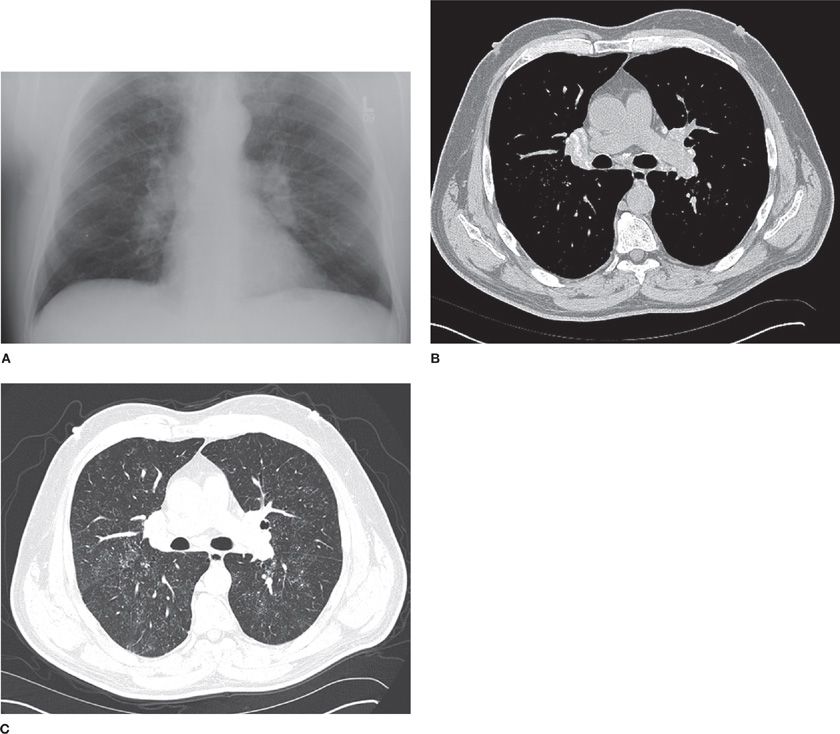
Radiographic changes in CBD are nonspecific and cannot be differentiated from sarcoidosis (Fig. 87-1). The most common radiographic abnormalities are diffuse round and reticular abnormalities.11,12 While most patients have both round and reticular nodules, opacities may be only round or only reticular. These opacities are usually present diffusely throughout the lungs but may be confined to the upper lobes. Hilar adenopathy similar to what is commonly observed in sarcoidosis may also be seen in up to 50% of cases. However, the large “potato type” node involvement is not seen. As the disease advances, radiologic evidence of scarring and retraction can be seen. The hila are retracted upward and conglomerate mass and emphysematous bullae may be present. Gross architectural distortion can occur from severe fibrosis. Pleural thickening can be seen in the presence of long-standing disease. In early disease, complete resolution of radiographic abnormalities can occur secondary to corticosteroid therapy and may recur as the corticosteroids are tapered.13 Complete spontaneous disappearance of the radiographic lesions has not been observed. The computed tomographic appearance of CBD includes upper lobe or diffuse fibrosis, pulmonary nodularity, and hilar and mediastinal adenopathy. However, in biopsy-proven CBD the computed tomographic findings may be normal or demonstrate ground-glass changes.
Figure 87-1 A 61-year-old male smoker who had worked at a beryllium processing facility for 20 years. He had a productive cough for 10 years but denied any shortness of breath. He had a positive response of both his blood and BAL cells to beryllium (stimulation index [SI] for blood beryllium lymphocyte proliferation test [BeLPT] = 13.8 [Nl < 3.0], SI for BAL BeLPT = 306 [Nl < 5.0]). His BAL also demonstrated a marked lymphocytosis (cell yield = 6 × 105 cells/mL [Nl < 3.0 × 105 cells/mL], lymphocytes = 55.2% [Nl <20%]). A. Chest radiograph demonstrating nodular interstitial disease with adenopathy. B. Chest CT mediastinal window demonstrating calcified bilateral hilar and mediastinal lymph nodes. C. Chest CT lung window demonstrating a diffuse fine nodular pattern of interstitial lung disease that was most prominent in the mid and upper lung zones.
 IMMUNOPATHOGENESIS
IMMUNOPATHOGENESIS
There are three important characteristics of CBD. First, this disease is usually associated with industrial exposure to beryllium. The only cases that have been described in nonindustrial workers have been in individuals who lived near beryllium plants and were either exposed to the airborne emissions from the plant or from family members who brought contaminated work clothes into the home.14,15 All other cases have been described in individuals who have been involved in the heating, grinding, abrading, or handling of beryllium metals, alloys, salts, or oxides. In addition, workers not directly handling beryllium may be exposed from processes occurring near them. Industrial hygienic practices today include efforts to remove potential airborne beryllium at the source to prevent beryllium from becoming airborne, limiting the number of workers with potential exposure to beryllium, limiting skin exposure, and trying to keep the airborne levels as low as possible.16 Recently, the Department of Energy has used 0.2 μg/m3 as an action level because of the repeated reports of CBD with possible exposure below the Occupational Safety and Health Administration (OSHA) recommended threshold level of 2 μg/m3/8 h shift.17
A second important characteristic of CBD is the long time interval or latency that occurs between initial exposure and the onset of disease.14 The average time to the onset of clinical symptoms is 10 years. This fact combined with the lack of a clear-cut dose–response relationship to CBD has hampered efforts to determine a safe level of beryllium. Thus, it is uncertain whether a peak exposure level or a total accumulated dose is more important for the development of CBD. Individuals have been described (i.e., secretaries with apparently little exposure) who have worked in industry for less than 1 year and yet still develop disease years to decades later.
A third important characteristic of CBD is that not all exposed workers will develop the disease. Only 1% to 8% of exposed workers will ever develop the disease.14 This percentage appears to have remained the same despite dramatic efforts by industry to reduce the potential exposure in their workers. This last characteristic of CBD is most likely due to a genetic predisposition.
The suspicion that an immunologic reaction to beryllium caused CBD was based on the following observations: (1) Beryllium painted on the skin (patch testing) could elicit delayed-type hypersensitivity reactions in patients with CBD.18 However, because of the concern that patch testing could sensitize individuals to beryllium, skin testing has not been widely used. (2) CBD was associated with “immunologic granuloma.” (3) Finally, animal studies demonstrated that a hypersensitivity could be demonstrated in animals and that this could be passed with cells.19
In vitro studies that simulated patch testing were developed in the 1970s and applied to patients with CBD.20,21 The blood cells from a large percent of patients with CBD had positive proliferative responses to beryllium. In addition, after stimulation with beryllium, blood cells from many patients with CBD could release the lymphokine, macrophage inhibition factor. However, all patients with CBD did not have positive responses with their blood cells.22
The confirmation that CBD was due to a cell-mediated response to beryllium came in the 1980s when cells harvested from the bronchoalveolar lavage fluid (BALF) from patients with CBD were examined.23 Not only was a marked increase in the number and percent of CD4+ T lymphocytes in the BALF noted, but also a positive proliferative response of bronchoalveolar lymphocytes to beryllium was observed. Positive proliferative responses to beryllium were observed in all cases of CBD and negative responses were noted in beryllium workers with biopsy-proven, non-beryllium lung disease, patients with sarcoidosis and no history of beryllium exposure, and normal volunteers.24,25 Not only did all patients with CBD have a positive proliferative response of their bronchoalveolar cells to beryllium, but this response was more pronounced in their lung cells than their blood cells. The accumulation of beryllium-specific cells in the lungs of patients with CBD has been confirmed and shown to be due to effector memory T cells26 that produce TH1 cytokines (IL-2, γ-interferon, TNFα).27,28 The T-cell response to beryllium is oligoclonal29 and the response can be blocked by anti-HLA DP antibodies and requires the presence of HLA-DP2 antigen presenting cells.30
The CD4+ T-cell response to beryllium suggested that specific HLA class II molecules might be involved in CBD, since HLA class II molecules present antigenic peptides to CD4+ T cells. A strong association of CBD with the marker HLA DPB1-glu 69 was first shown by Richeldi et al.31 and subsequently confirmed in three other laboratories.32–34 However, rather than just a marker for CBD, this marker appears to be associated with the ability to develop an immune response to beryllium. The less frequent alleles that code for DPB1-glu 69 appear to be more associated with beryllium sensitization and disease than the more common alleles.35,36 The crystal structure of HLA-DP2 has been solved37 and helps to explain the association with beryllium disease. βGlu 69 lies in a pocket that is accessible to beryllium. In addition, two additional acidic amino acids, βGlu 26 and βGlu 69, lie in this pocket. Mutation of any one of these three amino acids eliminated the ability of the molecule to present beryllium to sensitized T cells. A similar molecule on DR may be responsible for the development of beryllium sensitization in those who do not have HLA DPB1-glu 69.35 Additional genes probably account for the progression from beryllium sensitization to disease. Polymorphisms on a number of genes including IL-1 A,38 CC chemokine receptor 5,39 BTNL2 in DP glu 69 negative individuals,40 and TGFb141 have been identified as a possibility progression factor for the development of CBD. No association has been found with TNFα promoter regions42 or the TNFα gene.43
The previously mentioned studies suggest the following model for the pathogenesis of CBD. Beryllium is inhaled and deposited in the periphery of the lung. Beryllium, either alone as a crystal or combined with a normal lung protein(s), is bound by glu 69–containing DPB1 molecules and presented to berylliumspecific T cells. The beryllium protein or beryllium crystal is poorly digestible and cannot be removed by the immune response. Persistent inflammation leads to granuloma formation. The cells of the granuloma secrete enzymes that cause tissue destruction and fibrosis.
 DIAGNOSIS
DIAGNOSIS
Because of the frequent need for corticosteroid treatment of patients with CBD, all patients should have tissue confirmation of their diagnosis. A confirmed diagnosis of CBD requires demonstration of a granulomatous reaction secondary to beryllium hypersensitivity. The former requires biopsy material. The latter can be most convincingly demonstrated by testing the proliferative response of bronchoalveolar cells to beryllium. If bronchoalveolar lavage (BAL) cells cannot be easily or safely obtained, testing of blood proliferative responses to beryllium is a reasonable alternative. Laboratories performing these tests are listed in Table 87-1. In cases where biopsy demonstration of granulomatous inflammation is not possible, radiologic evidence of granulomatous inflammation may substitute.
Because immunologic tests of beryllium hypersensitivity have been available only since the late 1980s, their use for screening worker populations is not clear. However, studies to date indicate that blood proliferative response to beryllium is the most sensitive screening test for CBD. The major difficulty with using the blood proliferative response to beryllium as a screening tool is that not all individuals with beryllium sensitization will go on to develop CBD. In addition, the number of workers with beryllium sensitization that ultimately develop symptomatic CBD that requires treatment is unknown but may be relatively low8 and is probably less than 10%.
In addition, the justification for a screening test requires that there must be some action that will alter the course of the disease. Although it is generally believed that early treatment of CBD will alter the natural course of this condition, this is not certain.44 In addition, removal from further exposure, a prudent but unproven practice, is possible for current workers but would not be applicable to former workers. Thus, the strongest recommendation for use of the beryllium lymphocyte proliferation test as a screening test can be made for current workers. Recommendations for screening former workers and residents of communities with past beryllium exposure from the ambient air are less certain. Nevertheless, because the risk of developing CBD is lifelong, the question of appropriate screening for exposed individuals’ remains.
 DIFFERENTIAL DIAGNOSIS
DIFFERENTIAL DIAGNOSIS
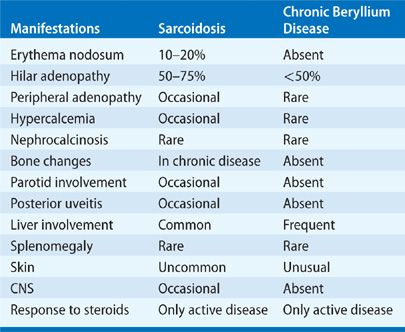
The major challenge to making the diagnosis of CBD is to think of the possibility of beryllium exposure. Most cases of CBD that are misdiagnosed are diagnosed as sarcoidosis because either the exposure to beryllium was not known by the patient or the physician failed to elicit an occupational history. In 84 cases of sarcoidosis with a history of possible beryllium exposure, a final diagnosis of CBD was made in 34 with a mean delay from the diagnosis of sarcoidosis of 4 years.45 A similar search for cases of beryllium disease in a cohort of sarcoidosis patients, failed to identify any cases of beryllium disease.46 Because the radiographic and clinical presentation of CBD (Table 87-2) is similar to sarcoidosis,47 the differential diagnosis includes upper-lobe fibrotic processes (Table 87-3). In addition, as for sarcoidosis, other causes of granulomatous disease must be searched for and eliminated. The differential between sarcoidosis and CBD depends upon the result of the proliferation test to beryllium. Patients with sarcoidosis do not respond to blood proliferation to beryllium, while CBD patients do. Cases of sarcoidosis among beryllium workers can be diagnosed in this manner. However, caution should always be used and repeatedly negative blood and lung tests should be determined before accepting a case of granulomatous lung disease in a beryllium worker as sarcoidosis.
 TREATMENT
TREATMENT
No standard approach to the use of corticosteroids has been adopted in the treatment of CBD.48–50
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree