Historical perspective
Exactly one century ago Carlos Chagas discovered an entirely new infectious entity – Chagas disease, its etiologic agent – Trypanosoma cruzi, and the main mechanism of transmission of the disease, through the sting of Triatominae bugs.1 By the early 1920s the most relevant syndrome of Chagas disease – cardiomyopathy – had been characterized, including its florid electrocardiographic disturbances.2 However, during subsequent decades the disease was practically neglected and its epidemiologic significance was even refuted. Only in the 1950s, with the completion of systematic serologic and necropsy studies, did it became apparent that T. cruzi infection was responsible for the triad of cardiomyopathy, megaesophagus and megacolon which afflicted millions of people in Latin America.3
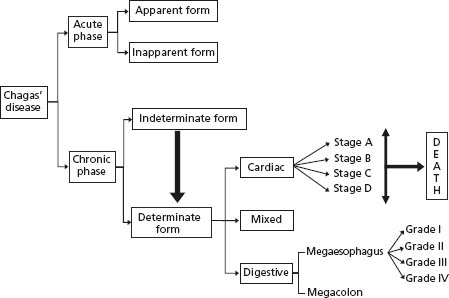
Chagas’ disease is characterized by acute and chronic phases. In the acute phase more than 90% of the patients have the unapparent form, while the remaining infected individuals show a febrile disease and rarely die because of myocarditis or encephalitis. During the chronic phase most patients remain for life with the indeterminate form. Others eventually show the cardiac, the digestive or the mixed form of organic involvement.4 Cardiac and esophageal chagasic disease is further classified in stages (grades) (Fig. 51.1).
Although megaesophagus and megacolon produce typical clinical conditions in roughly 5–10% of patients, chronic Chagas’ cardiomyopathy (CCC) is by far the most serious form of the disease.5
The long period of the indeterminate form – several decades before the appearance of clinically manifest disease – constitutes one of its most intriguing features.6 Its traditional definition by a specialist panel in 1984 requires that asymptomatic patients with positive specific serology show no physical signs of disease, and no abnormalities in the ECG and in the radiologic assessment of the heart, esophagus and colon (barium swallowing and enema).7 Although not adhering to such strict criteria (for instance, most studies do not allude to performing colon or esopha-geal X-rays) investigators using more sensitive diagnostic methods have described cardiac abnormalities in the majority of patients with the indeterminate form.8 More significantly, patients with normal ECG and chest X-rays may have striking alterations in right ventricular biopsy specimens9 and right ventricular contractile depression.10 Also, these patients may have mild segmental or global left ventricular (LV) dysfunction as evaluated by the slope of the end-systolic pressure-dimension relationship.11 In addition, independent of the clinical classification, the presence of minor LV wall motion abnormalities documented at baseline two-dimensional (2D) echocardiography in patients who have normal EKG and normal global systolic function is a predictor of deterioration of ventricular function during follow-up.12
Based on these findings, it has been proposed that seropositive chagasic patients who have minor LV wall motion abnormalities on 2D-echocardiography, despite normal ECG and chest X-rays, should be classified in the cardiac form of the disease. Conversely, patients with the indeterminate form of the disease should have normal global and segmental biventricular systolic function as documented by imaging techniques.13 Whether every chagasic patient with normal ECG and chest X-rays should routinely undergo other cardiac tests is a distinct and controversial issue. Because the probability of survival of these patients is similar to that of the normal population, the current predominant view is that further initial evaluation is usually unnecessary. Subsequent follow-up of chagasic patients with normal ECG should rely on annual history, physical examination and repeated ECG.14
Transmission of T. cruzi is mainly vectoral, through the feces of infected bloodsucking insects of the family Reduviidae (subfamily Triatominae). Many case series reports have documented that the infection can also occur by blood transfusion, transplacental transmission, laboratory accident, organ transplantation and ingestion of Triatominae-contaminated food or drink.
Although cross-sectional epidemiologic studies assessing clinical manifestations and mortality rates have been reported in scattered areas of several South American countries, the actual prevalence of CCC is unknown, because no recent large-scale screening has been carried out. However, it is unquestionable that the global disease prevalence has steadily reduced. Recent rough estimates are in the range of 8–10 million people who have Chagas’ disease in the Americas,15,16 contrasting with the 1990 estimates of 16–18 million people infected.
In 1991 an initiative for the elimination of the transmission of Chagas’ disease was launched in Argentina, Brazil, Bolivia, Chile, Paraguay and Uruguay, covering an area responsible for more than 65% of the global prevalence. As result, Uruguay (1997), Chile (1999) and Brazil (2006) declared themselves free of Chagas’ disease transmission due to Triatoma infestans.17 It is estimated that the incidence of new cases declined from 700 000/year in 1983 to 200 000/year in 2000.18 Other initiatives are now ongoing in other Latin American countries with results still pending. Nevertheless, CCC is by far the most common form of cardiomyopathy in Latin America countries. Further, because of modern migratory trends, it is likely to become ubiquitous.19 This tendency can be exemplified by estimates, based on the prevalence of T. cruzi infection detected serologically (mostly Latin American immigrants), that 100000–400000 infected persons might be living in the United States.20,21 The FDA recently issued strict surveillance of blood supply regarding T. cruzi infection, and in Europe, preventive measures have also been suggested.22
In the United States vector-borne transmission is rare23 but Chagas seropositivity incidence among blood donors is increasing.24 A recent survey by the American Red Cross, in facilities located in California and Arizona, identified 63 specimens from 61 donors among 148 969 blood donation specimens (nearly one in 2365 donations). Additionally, a systematic review of the literature providing evidence -based recommendations for the evaluation, counseling, and treatment of patients with chronic T. cruzi infection in the United States has recently been published.14
CCC has a high social impact.25,26 Over 15000 annual deaths can be attributed directly to this etiology and 667 000 disability-adjusted life years (DALYs) are lost annually.18 Chagas’ disease constitutes the third largest parasitic disease burden globally, after malaria and schistosomiasis.
Natural history and prognostic factors
There is sound experimental, pathologic, and clinical evidence that T. cruzi tissue parasitism produces organ damage acutely (immediately following infection) as well as decades later.3 Acute infection is often asymptomatic or may be a self-limited febrile illness lasting 4–8 weeks. The case fatality rate in the acute phase is less than 5%, death occurring because of myocarditis and/or meningoencephalitis. Following the acute phase 30–40% of chronically infected individuals develop the clinical manifestations of cardiac and/or digestive disease, the majority 10–30 years after the initial infection.
Cardiac involvement typically produces conduction defects, ventricular arrhythmias, wall motion abnormalities, cardiac failure, pulmonary and systemic thromboembolic phenomena, and sudden death.5 Megaesophagus and megacolon are also frequently diagnosed in chronic chagasic patients in Brazil, Argentina, and Chile, but not in Mexico, Colombia or Venezuela.4 The hypothesis that different T. cruzi strains, coupled to genetic susceptibilities of infected individuals and to environmental factors, may cause this difference in morbidity has not been evaluated by appropriately designed studies.27
The natural history of CCC is relatively well known from observational studies conducted mainly in endemic areas of Brazil, Argentina, and Venezuela since the early 1940s. There is also a wealth of case series reports dealing with acute Chagas’ disease acquired through non-vector transmission. Most of these investigations consist of longitudinal or cross-sectional observations of infected people in rural areas. Very few studies have been conducted using case–control populations of chagasic and non-chagasic people. There have also been some observational investigations focusing on the description and follow-up of hospital based cohorts.
Both the rural and the hospital-based studies have limitations. There is usually no adequate identification of cardiac involvement in the rural studies. Furthermore, because of the protracted course of heart involvement, from the acute phase to end-stage heart failure, no studies encompassing the whole span of the disease are available. Conversely, in hospital-based studies the disease is often well characterized but results could not be extended to the whole chagasic population. More recently, several longitudinal studies have been conducted on clinic-based populations at different referral centers in an attempt to establish predictors of death for patients with CCC.28 These studies have analyzed clinical, radiologic, electrocar-diographic, echocardiographic and Holter monitoring variables.
Prognosis in the acute phase
Case series using serologic tests in endemic areas have shown that in less than 10% of the acute cases were clinical manifestations sufficient to make a correct diagnosis.29 This is a major deterrent for understanding the transition from the acute to the chronic phases. However, the scarce clinical data are in general agreement with findings from experimental models of Chagas’ disease.
For the minority of patients in whom the clinical diagnosis was possible, cardiac involvement occurred in 90% of 313 successive cases reported in Bambuí (central Brazil). Of note, in 70–80%, cardiac enlargement was seen on chest X-rays, contrasting with only 50% of cases showing ECG abnormalities.29 This is in disagreement with other reports showing a predominance of ECG alterations.30 In the Bambu í study, the severity of myocarditis was inversely proportional to age, heart failure being more intense in children aged up to two years. Mortality in the acute phase, as seen in the 313 cases, was 8.3%. This was higher than the 3–5% reported in similar studies in other endemic areas in Brazil, Argentina, and Uruguay. The ECG was normal in 63.3% of the non-fatal cases and in only 14.3% of those who died in the acute phase. Seventy-five percent of all deaths were seen in children aged <3 years. Heart failure was the constant finding in all fatal cases, with or without concomitant encephalitis.29
The typical course of acutely manifested Chagas’ disease includes disappearance of symptoms and signs of heart failure within 1–3 months and normalization of the ECG after one year of the infection. However, there is no evidence of spontaneous cure of the infection, as demonstrated by serial xenodiagnosis and serologic tests in studies of several hundreds of chagasic patients. Of 117 patients from Bambuí who were followed for up to 40 years after the acute infection, the development of CCC (based on clinical signs, ECG, and chest X-ray) occurred in 33.8%, 39.3%, and 58.1% for follow-up periods of 10–20 years, 21–30 years, and 31–40 years respectively.29 In another review from the same cohort, for 268 patients whose acute phase of the disease had been diagnosed an average of 27 years before, the general mortality for the period was 13.8%.29
In contrast to the low mortality following the vectoral transmission, recent outbreaks of the disease, caused by ingestion of contaminated food or fluids, were associated with higher mortality rates, probably due to massive parasitic burden.31
Prognosis in the indeterminate form
The mechanisms responsible for preventing the progression of disease in more than 50% of cases (i.e. those with the indeterminate form) remain obscure. A 1–3% annual rate of appearance of CCC has been observed in several studies. Of 400 young adults followed for 10 years in S ã o Felipe (north eastern Brazil), 91 (23%) developed ECG or chest X-ray abnormalities. Of note, eight deaths were recorded in that period, seven of them due to non-chagasic causes and only one occurring because of reactivation of myocarditis.6
Another longitudinal study in Bambu í contrasted the evolution of 885 young patients with indeterminate form to that of 911 chagasic patients with initially abnormal ECGs. Survival after 10 years was 97.4% and 61.3% respectively for the indeterminate group and the group with CCC.32 Athird longitudinal study in a rural Venezuelan community, with 47% prevalence of positive serology for Chagas’ disease, followed 364 patients for a mean period of four years. Heart involvement appeared at a rate of 1.1% per year in seropositive individuals. Mortality was 3% in the four years of follow-up, CCC being the cause of death in 69% of all fatal cases.33
In 1973 a longitudinal study was initiated in Castro Alves (Bahia), a rural community in north east Brazil. In the initial cross-sectional study, of 644 individuals aged >10 years, 53.7% were seropositive. The population initially described in 1973–1974 was re-examined in 1977, 1980, and 1983. The overall rate of development of abnormal ECG was 2.57% in seropositive as compared to 1.25% per year in seronegative individuals, a relative risk of 2.0 for the same geographical area.34 These results were obtained in chagasic populations with >50% of the patients aged <20 years. It is relevant that fewer indeterminate cases are found in the older age groups because of the evolving nature of the disease (that is, more aging patients presenting clinical signs of cardiac or digestive involvement).
Another study of 160 patients with the indeterminate form was conducted in São Paulo, Brazil, from 1979 to 1994. Patients were followed long term with repeated electrocar-diographic and echocardiographic studies at regular intervals. After a mean follow-up of 8.2 years, 34 patients (21.3%) developed ECG changes. However, only in 15 of the 34 patients could the ECG changes be clearly attributed to CCC. In addition, LV ejection fraction remained normal in the entire population throughout the study, certainly contributing to the favorable outlook for these patients.35 More recently, a longitudinal study in Argentina following 731 patients (mean age 44 years) with the indeterminate form of Chagas’ disease for eight years found that 4.6% progressed to clinically manifest cardiomyopathy. Four patients (0.5%) died of cardiovascular causes during this period, but their cardiac status right before the death was not reported.36 Finally, in the larger series of 4593 chagasic patients followed for an average of 5.3 years in Argentina, those with normal ECG showed a very low sudden cardiac mortality rate (0.004% per year).37
Prognosis of chronic Chagas cardiomyopathy
The evidence provided by the studies mentioned above shows that chagasic individuals with a normal ECG, in general, have a very good prognosis. In contrast, the appearance of ECG changes entails an unfavorable prognosis. In a community-based study in rural Brazil, right bundle brunch block and ventricular extrasystoles were each associated with 7–8-fold increase, and the two together with a 13-fold increase in risk of death over a seven-year period, as compared to the infected population with normal ECG.38
In another study of 107 chagasic patients followed for 10 years, a significant reduction in life expectancy, as compared to that of 22 control individuals, was detected only in patients with ECG or clinical changes. A mortality rate of 82% over the 10-year follow-up period was seen in the group of 34 patients with signs of heart failure at the beginning of the study. In contrast, a 65% 10-year survival was associated with ECG abnormalities but in the absence of signs of heart failure.39 In a study of 160 cases, survival two years after the first episode of heart failure was only 33.4%.40 Another study of 104 male chagasic patients admitted to hospital with heart failure revealed a mortality rate of 52% after five years. The strongest predictors of survival were reduced LV ejection fraction and maximal oxygen uptake during exercise.41 The data support the concept that mortality associated with Chagas’ disease strongly correlates with severity of myocardial dysfunction.
In a series of 42 patients with CCC in the United States, death occurred in 11 patients during a mean follow-up of nearly five years, always in association with global or regional LV dysfunction. Established or developing heart failure was a strong predictor of mortality but aborted sudden death or the presence of sustained ventricular tachycardia were not predictors for mortality in this series.42 These results contrast with the evidence from 44 chagasic patients followed for a mean period of two years where ventricular tachycardia detected during exercise testing was a marker of increased risk of sudden death.43 This discrepancy probably reflects the limitations of small number of patients and relatively short follow-up in both studies.
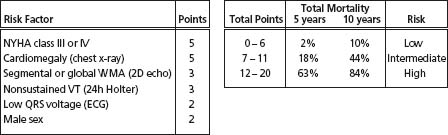
In a recent study of 424 patients with Chagas’ heart disease in central Brazil, a mortality risk score based on extensive follow-up data was developed and validated in an external sample, after univariate and multivariate analysis. Six independent predictors of death were identified44 (Fig. 51.2). Finally, a systematic review of all reports on prognostic factors using multivariate analysis in CCC confirmed that the risk of death is strongly related to the severity of disease, with markers of LV dysfunction, congestive heart failure and complex ventricular arrhythmias carrying the gravest prognosis.28
Figure 51.2 Risk score for predicting death in chronic Chagas cardiomyopathy. NYHA, New York Heart Association; WMA, wall motion abnormality; VT, ventricular tachycardia.
Key points
- As long as patients remain in the indeterminate form, their prognosis is good. Level C1
- After 10 years almost 80% of patients remain with the indeterminate form of the disease and probably 50% of the entire population will have no signs of heart disease throughout their life span. Level C1
- There are no clues as to why some chagasic patients remain with the indeterminate form, while others sooner or later undergo severe organic damage.
- The prognosis of patients with Chagas’ heart disease is predictable, despite the heterogeneity of clinical manifestations of the disease. Level C1
- By univariate analysis many variables indicate a poor prognosis. However, taking into account the relationship among variables (multivariate analysis), impaired LV dysfunction, NYHA class III/ IV, cardiomegaly, and non-sustained ventricular tachycardia are consistently and independently associated with higher mortality. Level C1
- The combination of non-sustained ventricular tachycardia (NSVT) and LV dysfunction is associated with a substantial increased risk of mortality when compared with patients without any of these risk markers. Level C1
- Once overt cardiac failure ensues the prognosis is poor and approaches 50% in four years. Level C1
- It is possible, but not proven by good evidence, that ventricular dysrhythmia and sudden death play a more prominent role in mortality due to Chagas’ disease than in heart failure due to other etiologies. Level C2
Pathophysiology and pathogenetic mechanisms
Organ damage arising during the acute phase is closely related to parasite presence in targets such as the gastrointestinal tract, central nervous system and heart. High-grade parasitemia co-exists with lymphadenopathy, liver and spleen enlargement, markers of widespread immunologic reaction. As the parasitemia abates and the systemic inflammatory reaction subsides, silent relentless focal myocarditis ensues during the indeterminate phase.45 In predisposed hosts, encompassing approximately 30–50% of the infected population, this chronic myocarditis may evolve to cumulative destruction of cardiac fibers and marked reparative fibrosis. The incessant myocarditis is eventually responsible for myocardial mass loss attaining critical degrees, thereby leading to regional and global ventricular remodeling and chamber dilation, and setting the anatomic substrate for malignant dysrhythmias. This hypothesis is based on several experimental animal models for CCC, but additional evidence has been provided by studies correlating clinical and pathologic findings in autopsied humans dying with all forms and stages of the disease.
The most intriguing challenge for understanding the pathogenesis of CCC lies in the complex host –parasite inter-relationship. In many patients, the myocardial aggression is controlled at low levels, remaining with the indeterminate form, whereas in others the development of fully blown CCC is triggered.46
Evidence from pathophysiologic studies in animal models and in humans points to four pathogenetic mechanisms producing myocardial damage in CCC.45
1. Cardiac autonomic disturbances
Intense neuronal depopulation has been demonstrated in several independent pathologic studies.47,48 Also, abnormal autonomic cardiac regulation has been shown in many functional investigations, preceding the development of ventricular dysfunction.10,49 Because of the dysautonomia, chagasic patients are deprived of the tonic inhibitory action normally exerted by the parasympathetic system on the sinus node, and also lack the vagally mediated mechanism to respond with rapid bradycardia or tachycardia to transient changes in blood pressure or venous return.50,51 Although sympathetic denervation has also been shown in myocardial regions, even preceding perfusion defects and wall motion abnormalities,52 on the basis of the striking parasympathetic impairment, the neurogenic theory postulated that a long-lasting autonomic imbalance would lead to a catecholamine-induced cardiomyopathy.47,53
However, various kinds of evidence militate against neurogenic derangements being a main pathogenetic mechanism.45 First, even in endemic areas where cardiac denervation is readily detectable in some patients, its prevalence and intensity are highly variable. Second, no significant correlation seems to exist between parasympathetic denervation and the extent of myocardial dysfunction or the presence of dysrhythmia. Moreover, the typical chagasic cardiomyopathy is found in geographical regions where the disease is apparently caused by parasite strains devoid of neurotropism.4 In such regions the typical chagasic digestive syndromes considered to be causally related to parasympathetic denervation of the esophagus and colon are rarely described. Third, no follow-up studies correlating autonomic regulation, myocardial function, and cardiac rhythm assessment have been reported in chagasic patients. Finally, the attractive hypothesis of autonomic impairment triggering sudden death has never been appropriately tested.
2. Microvascular disturbances
There is evidence from studies in experimental models of T. cruzi infection, as well as from pathologic and clinical investigations, that microvascular abnormalities causing ischemia contribute to the pathogenesis of CCC. In the experimental murine model of Chagas’ disease, occlusive platelet thrombi in small epicardial and microcirculatory coronary vessels have been shown in concomitance with focal vascular constriction and spasm.54,55 These abnormalities were partly reverted by long-term administration of verapamil, leading to attenuation of the myocardial damage and increased survival for the T. cruzi infected mice.56
Biopsy and autopsy histopathologic investigations in humans also showed microvascular abnormalities deemed responsible for the focal diffuse myocytolysis and extensive reparative fibrosis known to be related to transient ischemic disturbances of low intensity and short duration.3,47,57–59
From the clinical standpoint most chagasic patients have ischemic-like symptoms and ST changes and abnormal Q-waves indicative of regional myocardial fibrosis. They also have prominent LV localized dysynergy and striking myocardial perfusion defects in the absence of epicardial coronary artery obstruction.60 Of note, these reversible isch-emic defects are mostly seen in the apical and inferior-posterior LV segments, i.e. the regions where regional contractile dysfunction prevails in later stages of CCC.52 In a recent study, 36 chagasic patients with normal coronary arteries were sequentially evaluated regarding progression of left ventricular dysfunction and myocardial perfusion disturbances over a mean period of 5.6 years. The results showed that the decrease of left ventricular ejection fraction over time was correlated to the increase of regional myocardial perfusion at rest, which is indicative of myocardial fibrosis. The appearance of new perfusion defects at rest at the final evaluation was topographically related to the presence of reversible ischemic perfusion defects at the initial scan.61 Thus, microcirculatory ischemia could represent an ancillary factor to potentiate and amplify the chronic inflammatory aggression to myocardial tissue, with coalescent microinfarctions leading to the development of aneurysms.45
3. Myocardial damage directly related to parasite persistence
Early in the natural history of CCC there is a low-grade but constant focal and diffuse inflammatory process, as shown by studies in experimental models62 and in human necropsy (chagasics dead from other causes) and biopsy specimens.9,63,64 Several independent investigators using immunohistochemistry, PCR and in situ hybridization methods described tissue T. cruzi antigens or its genomic material in the inflammatory foci, first in animal models64,65 and later in humans.65–70 There is also evidence of a direct correlation between organic parasite infection and the clinical expression of disease. Thus, T. cruzi genetic material could not be detected in the heart from patients who died without signs of cardiac involvement, but it was consistently found in heart specimens from patients with CCC.69 Moreover, the persistence of T. cruzi is directly implicated in the pathology of the chronic phase, as shown by experimental models of Chagas’ disease in which parasite load reduction by trypanocidal treatment resulted in attenuation of cardiomyopathy71,72 Conversely, in experimental models, enhancement of parasite burden results in exacerbation of the cardiomyopathy course.73 Finally, in human CCC, during favorable conditions such as immunosuppression treatment to prevent transplant rejection or in patients with acquired immunodeficiency syndromes, intact amastigote parasites undertake active multiplication and cause reactivation of infection and striking recrudescence of myocarditis and esophagitis.74–79
4. Immune-mediated cardiac damage
Immunologic factors have been postulated to play a role in the pathogenesis of CCC. The demonstration of predominant mononuclear cells in the inflammatory infiltrates suggests a delayed hypersensitivity reaction, leading to immunoglobulin and complement deposition in myocardial tissue.80,81 Experimental and clinical data from studies in CCC also show that several of the criteria required for an autoimmune pathogenesis have been fulfilled:82
- the identification of heart–T. cruzi cell cross-reactive T cell antigens with reproduction of pathobiologic changes by passive transfer of immune cells in murine models
- attenuation of the inflammatory process as a consequence of tolerance induction to myocardial antigens
- the induction of myocardial aggression after immunization with cardiac myosin
- the isolation of cardiac myosin autoreactive T cells in molecular mimicry with T. cruzi B13 protein from affected tissue
- in vitro immunization with B13 protein eliciting T cell clones cross-reactive with cardiac myosin
- immunization with T. cruzi ribosomal antigens inducing cross-reactive antibodies and heart conduction abnormalities
- similar cross-reactive autoantibodies present in sera from patients with CCC disease inducing arrhythmia in explanted hearts.
- In summary there is persuasive evidence of both parasite antigen-driven immunopathology and autoimmunity in cardiac inflammatory lesions. However, the nature of the essential antigen or antigens that elicit destructive immune responses remains elusive.45
Key points
- Cardiac dysautonomia is a well-characterized feature preceding myocardial damage, but its role is probably ancillary in the pathogenesis of CCC. Level B
- Microcirculatory derangements causing ischemia probably constitute important amplification mechanisms for the inflammatory myocardial aggression. Level B
- The low-grade but incessant myocarditis present in CCC is inextricably related to the parasite persistence, with superimposed antiparasite immunity and autoimmune reaction, but the mechanisms triggering exacerbated responses in some cases and deterring significant damage in others still remain to be elucidated. Level B
- A corollary of the parasite persistence hypothesis, with potential clinical implication, is that effective measures to control the parasite burden would probably reduce tissue damage and impact on the natural history of CCC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree