Cerebrovascular Disease and Neurologic Manifestations of Heart Disease: Introduction
Brain and Cerebrovascular Complications of Heart Disease
Stroke is a common and devastating disease, the third leading cause of death and the leading cause of disability in the United States. Cardiogenic stroke can occur when (1) the heart pumps unwanted materials into the circulation that reach the brain (embolism), (2) pump function fails and the brain is hypoperfused, and (3) drugs given to treat cardiac disease have neurologic adverse effects.
Cardiogenic cerebral embolism is responsible for approximately 20% of ischemic strokes.1-5 However, because many patients have coexisting cardiac and extracranial vascular disease,5 criteria for the diagnosis of cardiac embolism remain controversial even today. As more advanced diagnostic techniques have been developed, more causative cardiac abnormalities (and their association with stroke) have been recognized. Cardiac sources of brain emboli can be divided into three groups6:
Cardiac wall and chamber abnormalities: cardiomyopathies, hypokinetic and akinetic ventricular regions after myocardial infarction (MI), atrial septal aneurysms, ventricular aneurysms, atrial myxomas, papillary fibroelastomas and other tumors, septal defects, and patent foramen ovale
Valve disorders: rheumatic mitral and aortic disease, prosthetic valves, bacterial endocarditis, fibrous and fibrinous endocardial lesions, mitral valve prolapse, and mitral annulus calcification
Arrhythmias: especially atrial fibrillation (AF) and sick sinus syndrome
Some cardiac sources have much higher rates of initial and recurrent embolism. The Stroke Data Bank7 divided potential sources into strong sources (prosthetic valves, AF, sick sinus syndrome, ventricular aneurysm, akinetic segments, mural thrombi, cardiomyopathy, and diffuse ventricular hypokinesia) and weak sources (myocardial infarct >6 months old, aortic and mitral stenosis and regurgitation, congestive failure, mitral valve prolapse, mitral annulus calcification, and hypokinetic ventricular segments). The risk of embolism varies within individual cardiac abnormalities depending on many factors. For example, in patients with AF, associated heart disease, patient age, duration, chronic versus intermittent fibrillation, and atrial size all influence embolic risk. The presence of a potential cardiac source of embolism does not mean that a stroke was caused by an embolus from the heart. Coexistent occlusive cerebrovascular disease is common. In the Lausanne Stroke Registry, among patients with potential cardiac embolic sources, 11% of patients had severe cervicocranial vascular occlusive disease (>75% stenosis), and 40% had mild to moderate stenosis proximal to brain infarcts.5
Persistent and paroxysmal AF is a potent predictor of first and recurrent stroke, with >75,000 attributed cases annually. In patients with brain emboli caused by a cardiac source, there is a history of nonvalvular AF in roughly one half of all cases, of left ventricular thrombus in almost one third, and of valvular heart disease in one fourth.1,8 Stroke prevention in patients with AF and other heart diseases will be discussed later on in this chapter.
Intracavitary thrombus caused by acute MI occurs in an estimated one-third of patients within the first 2 weeks after anterior MI and in an even greater proportion of patients with large left ventricular apex infarcts.2,8 Ventricular thrombi can also occur in patients with chronic ventricular dysfunction caused by coronary disease, hypertension, and dilated cardiomyopathy. Stroke is less common among uncomplicated MI patients but can occur in up to 12% of patients with acute MI complicated by a left ventricular thrombus. The rate of stroke is higher in patients with anterior rather than inferior infarcts and may reach up to 20% in those with large anteroseptal MI. The incidence of embolism is highest during the period of active thrombus formation in the first 1 to 3 months, with substantial risk remaining even beyond the acute phase in patients with persistent myocardial dysfunction, congestive heart failure, or AF.8,9
Congestive heart failure affects >4 million Americans and increases stroke risk by a factor of 2 to 3, accounting for an estimated 10% of ischemic strokes.3,8 In patients with nonischemic dilated cardiomyopathy, the rate of stroke is similar to that of cardiomyopathy caused by ischemic heart disease. An estimated 72,000 initial strokes annually are associated with left ventricular systolic dysfunction, and the 5-year recurrent stroke rate in patients with cardiac failure has been reported as high as 45%.8,10
Recurrent embolism occurs in 30% to 60% of patients with rheumatic mitral valve disease and a history of a previous embolic event.8,11-14 Between 60% and 65% of these recurrences develop the first year, many within the first 6 months.8,11,12 Mitral valvuloplasty does not appear to eliminate the risk of embolism.8,15,16
Mitral valve prolapse (MVP) is the most common form of valve disease in adults and is generally benign.17,18 MVP as a source of embolic stroke continues to be controversial.6 Several small clinical series have reported cerebral embolism in MVP patients who lacked other possible embolic sources.19-22 Patients with MVP also may have other disorders such as AF, syncope, and migraine. The rate of recurrent stroke in patients with MVP as the only known cause is very low.21,22
Mitral annulus calcification (MAC) is an important, often unrecognized cause of embolism. Several series show a convincing relation between MAC and brain emboli and stroke.6,23-25 Bacterial endocarditis can also develop on the MAC. Anticoagulation does not prevent calcific emboli. The decision to use antiplatelet agents versus anticoagulants should include consideration of other potential comorbid factors such as AF, which can occur 12 times more often in patients with MAC than it would in those without MAC.18,26
Aortic valve disease, in isolation, is not often associated with systemic embolism. Although there are isolated case reports of patients who had strokes from spontaneous aortic valve calcific emboli, few trials of selected patients with stroke and aortic valve disease exist at present.8,27 One prospective analysis of 815 patients with calcification of the aortic valve (with or without stenosis) did not show any association between either of the two aortic valvular lesions and stroke.28 Current treatment recommendations in these cases are based on larger antiplatelet trials of stroke and transient ischemic attack (TIA) patients.8
More patients may have cardiogenic embolism than are now diagnosed. Clinical features and brain investigations such as computed tomography (CT), magnetic resonance imaging (MRI), and angiography (CT, magnetic resonance [MR], and digital subtraction angiography) may suggest emboli, but often a clear source is unidentified. These cases, which are termed infarcts of unknown causes in the Stroke Data Bank,29-31 include as many as 40% of patients.
Fibrous and fibrinous lesions of the heart valves and endocardium are associated with certain medical conditions.6 Valve lesions occur in patients with systemic lupus erythematosus (Libman-Sacks endocarditis32), antiphospholipid antibody syndrome,33 and cancer and other debilitating diseases (nonbacterial thrombotic endocarditis). Mobile fibrous strands are also often found during echocardiography.6,34-36 Fibrin-platelet aggregates may attach to these fibrous and fibrinous lesions.
Embolic complications are common in patients who have infective endocarditis.6,37 Mycotic aneurysms can cause fatal subarachnoid bleeding. Bleeding can also result from vascular necrosis as a result of an infected embolus.37 Embolization usually stops when infection is controlled.34 Warfarin does not prevent embolization and is contraindicated unless there are other important lesions such as prosthetic valves or pulmonary embolism. In children and young adults with congenital heart defects, especially those with right-to-left shunts and polycythemia, brain abscess is an important complication.
Emboli often arise from sources other than the heart, such as the aorta, proximal arteries (intra-arterial or so-called local embolism), leg veins (paradoxical emboli), fat in the liver or bones (fat embolism), and materials introduced by the patient or physician (drug particles or air).6 The types of embolic material vary (Table 107–1).6,38Atheromatous plaques in the aortic arch and ascendingaorta are an important source of embolism to the brain (Figs. 107–1 and 107–2). Ulcerated atheromatous plaques are often found at necropsy in patients with ischemic strokes, especially in those in whom the stroke etiology was not determined during life.39 Transesophageal echocardiography (TEE) often shows these atheromas, but technical factors limit visualization of the entire arch.40 Large (>4 mm), protruding mobile aortic atheromas are especially likely to cause embolic strokes and are associated with a high rate of recurrent strokes.41,42 Use of oral anticoagulants rather than antiplatelet agents is recommended in these patients.18,43,44
| Cardiac | Intra-Arterial |
|---|---|
1. Red fibrin-dependent thrombi 2. White platelet-fibrin nidi 3. Material from marantic endocarditis 4. Bacteria from vegetations 5. Calcium from valves and mitral annulus calcification 6. Myxoma cells and debris | 1. Red fibrin-dependent thrombi 2. White platelet-fibrin nidi 3. Combined fibrin-platelet and fibrin-dependent clots 4. Cholesterol crystals 5. Atheromatous plaque debris 6. Calcium from vascular calcifications 7. Air 8. Mucin from tumors 9. Talc or microcrystalline cellulose from injected drugs |
Figure 107–1.
Descending aorta at necropsy from a patient whose transesophageal echocardiography before surgery showed severe disease of the ascending aorta and aortic arch with mobile protruding plaques. This patient died after coronary artery bypass grafting surgery having never awakened after the procedure. Courtesy Denise Barbut, MD, Cornell University Medical College and the New York Hospital.
Warning signs of stroke can include sudden hemiparesis, hemisensory loss, confusion, trouble speaking or understanding, visual loss, diplopia, ataxia, vertigo, or sudden severe headache with no known cause. Most embolic events occur during activities of daily living, but some embolic strokes have their onset during rest or sleep. Sudden coughing, sneezing, or arising at night to urinate can precipitate embolism.6,45 Although the deficit is most often maximal at outset, 11% of embolic stroke patients in the Harvard Stroke Registry had a stuttering or stepwise course, whereas 10% had fluctuations or progressive deficits. Later progression, if it occurs, is usually within the first 48 hours. Progression is usually caused by distal passage of emboli. Nonsudden embolism is explained by an embolus moving from its initial location, as demonstrated by angiography, to a more distal branch.6,46 Early angiography has a very high rate of showing intracranial emboli,29,47 but angiography after 48 hours shows a much lower rate of blockage. More recently, transcranial Doppler (TCD) sonography has shown a high incidence of middle cerebral artery (MCA) blockage acutely in patients with sudden-onset hemispheric strokes; but later, recanalization of the MCA and normalization of the intracranial blood velocities occur.6,48 As in all large infarcts, brain edema and swelling may develop during the 24 to 72 hours after stroke with headache, decreased alertness, and worsening of neurologic signs. The edema is often cytotoxic (inside cells) and usually does not respond to corticosteroid treatment.
Emboli usually cause occlusion of distal branches and produce surface infarcts that are roughly triangular, with the apex of the triangle pointing inward. CT and MRI findings can suggest the presence of embolism by the location and shape of the lesion,49 presence of superficial wedge-shaped infarcts in multiple different vascular territories, and hemorrhagic infarction, as well as visualization of thrombi within arteries. Among 60 patients with cardiogenic sources of embolism studied by CT in whom occlusive atherosclerotic cerebrovascular disease had been excluded, 56 had superficial large or small cortical or subcortical infarcts and only 4 had deep infarcts.49 Emboli can block the MCA and occasionally cause solely deep infarcts because the superficial territory has good collateral flow; these infarcts are called striatocapsular because they involve the internal capsule and the adjacent basal ganglia.6,45,50 Tiny emboli may cause small deep or superficial infarcts.
MRI, particularly with the use of MR diffusion-weighted and MR gradient recall echo (GRE) imaging, is more sensitive for detection of acute brain infarcts than is CT and is also superior in detecting hemorrhagic infarction by imaging hemosiderin. Hemorrhagic infarction has long been considered characteristic of embolism, especially when the artery leading to the infarct is patent.51 The mechanism of hemorrhagic infarction is reperfusion of ischemic zones after iatrogenic opening of an occluded artery (eg, endarterectomy, fibrinolytic treatment) or after restoration of the circulation after a period of systemic hypoperfusion. Hemorrhage occurs into proximal reperfused regions of brain infarcts.6,45,52 At times, it is also possible to image the acute embolus on CT and also via MRI with T2*-weighted gradient echo imaging.6,53-55
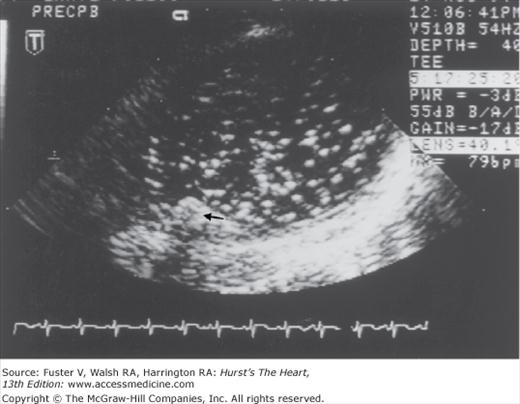
In unselected series of stroke patients, transthoracic echocardiography (TTE) has been variably useful in detecting sources.6,56-58 TTE is useful in patients with known cardiac disease to clarify potential embolic sources and heart function,45 in young patients without stroke risk factors, and in stroke patients who do not have lacunar infarction or ultrasound evidence of intrinsic atherostenosis of a major extracranial and intracranial artery. TEE provides much better visualization of the aorta, atria, cardiac valves, and septal regions. Reports of TEE suggest that the diagnostic yield is 2 to 10 times that of TTE.59-62 Aortic plaques, atrial septal aneurysms, and atrial septal defects are also much better seen with TEE (Fig. 107–3). The use of an echo-enhancing agent such as agitated saline helps detect intracardiac shunts.
Figure 107–3.
Transesophageal echocardiography recording during cardiac surgery from the aorta at the level of the origin of the left subclavian artery. A mobile plaque is seen protruding into the aortic lumen (small black arrow). This recording was taken after the release of aortic clamps and shows a shower of emboli within the aortic lumen beyond where the aorta was previously clamped. Courtesy Denise Barbut, MD, Cornell University Medical College and the New York Hospital.
Echocardiography has definite limitations. Particles the size of 2 mm can block major brain arteries but are beyond the imaging resolution of current echocardiographic technology.63 Also, thromboembolism is a dynamic process. When a clot forms in the heart and embolizes, there may be no residual evidence unless a clot reforms.6,38 Cardiac thrombi are imaged differently on sequential echocardiograms6,64; even large thrombi seen on one echocardiogram can disappear later.64
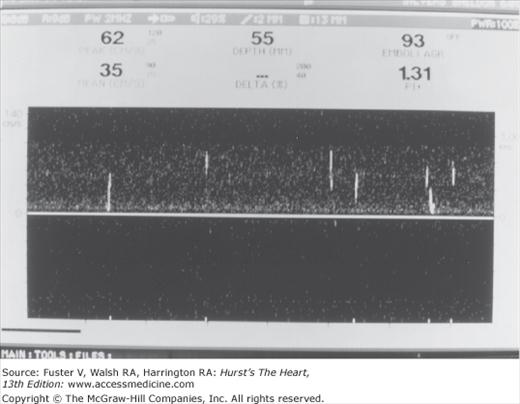
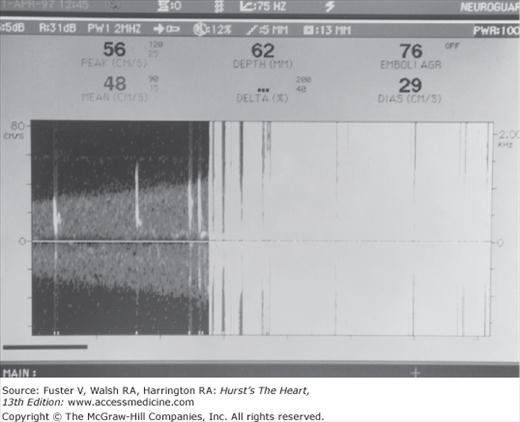
Cerebral embolic signals are now detected by monitoring with TCD.6,65,66 Embolic particles passing under TCD probes produce transient, short-duration, high-intensity signals referred to as HITSs (high-intensity transient signals). Examples of HITSs are shown in Figs. 107–4 and 107–5. TCD monitoring of patients with AF,67 cardiac surgery,68 prosthetic valves, left ventricular assist devices,69 carotid artery disease, and carotid endarterectomy and of stroke patients with a patent foramen ovale have shown a relatively high frequency of embolic signals.70 Monitoring of emboli with TCD may help guide treatment decisions.
Figure 107–5.
Transcranial Doppler recording from the middle cerebral arteries during cardiac bypass surgery. A few distinct emboli (white streaks in the left of the figure) are followed by a massive shower of emboli (whiteout) at the time of the release of aortic clamps. Courtesy Denise Barbut, MD, Cornell University Medical College and the New York Hospital.
Early studies showed that warfarin was effective in preventing brain embolism in patients with both rheumatic mitral stenosis and AF. Previously, the intensity of anticoagulation was higher than that currently used, and brain hemorrhages and other bleeding complications were common. Trials have now shown that lower dose warfarin (international normalized ratio [INR] 2.0-3.0) is also effective in preventing brain emboli in patients with nonrheumatic AF.
In the Copenhagen Atrial Fibrillation, Aspirin, Anticoagulation (AFASAK) study, 1007 patients (median age, 74.2 years) with chronic, nonrheumatic AF were assigned to warfarin (INR 2.8-4.2), aspirin (75 mg/d), or placebo.71 The study was halted prematurely when analysis of effectiveness reached a predetermined level of significance in favor of warfarin treatment. The principal outcome was the composite of ischemic or hemorrhagic stroke, TIA, and systemic embolism. The observed reduction for warfarin compared with placebo was 64%, an absolute risk reduction of 3.5% per year. An analysis by intention to treat, which excluded TIA and minor stroke, indicated a risk reduction of approximately 50% (P < .05) and an absolute reduction of approximately 1.5% per year.
The Stroke Prevention in Atrial Fibrillation (SPAF) study investigators evaluated warfarin and aspirin in patients with nonrheumatic AF.72,73 The study evaluated two groups of patients based on their eligibility for warfarin. In the first group, 627 patients judged eligible for warfarin were randomized to open-label warfarin (INR 2.8-4.5; prothrombin time [PT], 1.3-1.8 times control) or, in a double-blinded fashion, to either aspirin (325 mg daily, enteric coated) or a matching placebo. In the second group, 703 patients ineligible for warfarin were randomized (double blind) to aspirin (325 mg daily, enteric coated) or placebo. The principal outcome, a composite of ischemic stroke and systemic embolism, was significantly decreased during a mean follow-up of 1.3 years. The outcome of disabling ischemic stroke or vascular death was reduced by warfarin by 54% (P = .11), an absolute reduction of 2.6% per year. The outcome of disabling stroke or death was reduced 22% by aspirin (P = .33), an absolute reduction of approximately 1% per year. The SPAF investigators later compared low-intensity, fixed-dose warfarin (INR 1.2-1.5) plus aspirin (325 mg/d) with adjusted-dose warfarin (INR 2.0-3.0) in elderly patients with one or more risk factors for embolism.74 Ischemic stroke and systemic embolism were present in 7.9% of patients on fixed-dose warfarin plus aspirin versus only 1.9% of patients on adjusted-dose warfarin. SPAF investigators later studied the effectiveness of aspirin 325 mg in patients with low risk and found that the rate of ischemic stroke was low (2% per year).75
The SPAF study identified three risk factors for thromboembolism: (1) recent congestive heart failure, (2) history of hypertension, and (3) previous thromboembolism.76,77 The study also suggested that anticoagulation with warfarin was not indicated in patients with none of the three risk factors who were at low risk for thromboembolism (2.5% per year). In such patients, the dangers of anticoagulant therapy may outweigh its benefits. Aspirin (325 mg daily) is probably reasonable and safe therapy for patients with lone, nonrheumatic AF who are younger than 60 years of age and have none of the three identified risk factors.76-78 In all other patients with AF, long-term oral warfarin therapy (INR 2.0-3.0) should be used unless contraindicated.75,78,79
In the Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF), 420 patients with nonrheumatic AF (mean age, 68 years) were randomized unblinded to warfarin (target PT ratio, 1.2:1.5 × control; INR 1.5-2.7) or to a control group who were allowed to take aspirin.80 The principal outcome was ischemic stroke or systemic embolism, and the mean follow-up time was 2.2 years. The incidence of stroke was reduced by 86% in the warfarin group compared with the control group (P = .002), equivalent to an absolute risk reduction of 2.6% per year. There was no demonstrable benefit of aspirin, but the study was not designed to test aspirin.
In the Canadian Atrial Fibrillation Anticoagulation (CAFA) study, 187 patients were randomized to warfarin (INR target range 2.0-3.0), and 191 were assigned to placebo.81 The principal outcome was the composite of nonlacunar stroke, non-CNS embolism, and fatal or intracranial hemorrhage. The relative risk reduction for warfarin was 37% (P = .17). The study was ended prematurely when the results of the Copenhagen AFASAK and SPAF studies became known.
The European Atrial Fibrillation Trial (EAFT) Study Group addressed the question of the optimal level of anticoagulation by reviewing the results of their own trial.82 No treatment effect was found with anticoagulation responses less than INRs of 2.0. The rate of thromboembolic events was lowest at INRs from 2.0 to 3.9; most major hemorrhages occurred at INRs of 5.0 and above. The EAFT group recommended a target INR of 3.0, with a range from 2.0 to 5.0.82 Fixed-dose warfarin with a target INR of 1.3 to 1.5 was not as effective as standard adjusted-dose warfarin at an average INR of 2.4, even when aspirin 325 mg/d was added to the low fixed-dose warfarin in another study.
Although many trials have demonstrated the superiority of adjusted-dose warfarin over antiplatelet therapy for stroke prevention, participants in those trials tended to be younger (typically 70 years old) than the AF patients commonly encountered in clinical practice (typically late 70s with a substantial fraction of octogenarians). The Birmingham Atrial Fibrillation Treatment in the Aged (BAFTA) trial addressed this issue, randomizing 973 AF patients with age ≥75 years to adjusted-dose warfarin versus aspirin 75 mg/d. The stroke rate was 5% on aspirin and nearly halved by warfarin. Surprisingly, major hemorrhage rates were similar in both groups. It is of note that 40% of patients had received warfarin previously, potentially biasing toward lower bleeding rates. The investigators concluded that “these data lend support to the use of anticoagulation for all people aged over 75 years who have AF, unless there are contraindications or the patient decides that the size of the benefit is not worth the inconvenience of treatment.”83,84
Warfarin is approximately 50% more effective than aspirin in preventing stroke in patients with AF who do not have valvular disease. Data suggest that the optimal intensity of oral anticoagulation for stroke prevention in patients with AF appears to be a target INR of 2.0 to 3.0. However, the narrow therapeutic margin of warfarin, in addition to associated food and drug interactions, requires frequent INR testing and dosage adjustments. These liabilities likely contribute to underuse of warfarin, and alternative therapies are needed.
The Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE) evaluated the safety and efficacy of the combination of aspirin plus clopidogrel in AF patients who were unsuitable candidates for vitamin K antagonist therapy. In those patients, the addition of clopidogrel to aspirin did reduce the risk of major vascular events, especially stroke, but also increased the risk of major hemorrhage.85
Ximelagatran is a direct thrombin inhibitor that is orally administered, has stable pharmacokinetics independent of the hepatic P450 enzyme system, and has a low potential for food and drug interactions. Two large studies, Stroke Prevention Using the Oral Thrombin Inhibitor in Atrial Fibrillation (SPORTIF) III and V compared fixed-dose ximelagatran (36 mg twice a day) with dose-adjusted warfarin (INR 2.0-3.0) in 7329 high-risk patients with AF. In both trials, ximelagatran was noninferior to warfarin and had fewer major and minor bleeding complications. However, serum alanine aminotransferase levels increased transiently to >3 times normal in 6% of patients with ximelagatran (usually within the first 6 months). At the time of this publication, the US Food and Drug Administration (FDA) has not yet approved ximelagatran.8,86
Dabigatran is a different oral direct thrombin inhibitor that has also been compared with warfarin in a noninferiority trial. In this trial, 18,113 patients with AF were assigned to dabigatran 110 mg twice a day, dabigatran 150 mg twice a day, or adjusted-dose warfarin. Dabigatran was found, at the 110-mg dose, to have similar rates of stroke and systemic embolism as well as lower rates of major hemorrhage. The 150-mg dose had lower rates of stroke and embolism but similar rates of major hemorrhage. However, the rate of MI was higher in both dabigatran groups. Theoretically, if dabigatran were FDA approved, potentially the dose could be tailored to take into account a specific patient’s risk factors (although this concept was not tested by the trial).87
Other agents are being tested as well. For example, the ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) study is a prospective, randomized, double-blind, multicenter, event-driven, noninferiority study comparing the efficacy and safety of rivaroxaban, an oral, once-daily, direct factor Xa inhibitor with adjusted-dose warfarin in patients with nonvalvular AF. Additionally, a different factor Xa inhibitor, apixaban, is also being compared with warfarin in a similar ongoing trial.
The effectiveness of anticoagulation on embolic stroke prevention from other cardiac conditions has not been well studied. The concurrent use of aspirin plus warfarin in MI patients with left ventricular thrombus is based on American College of Cardiology/American Heart Association (ACC/AHA) guidelines for patients with ST-segment elevation MI.8,88 The rate of recurrent stroke in patients with MVP is so low that warfarin is not recommended for prophylaxis except when a thrombus is seen on echocardiography. Warfarin may not be effective in preventing calcific, myxomatous, bacterial, and fibrin-platelet emboli, and warfarin has been posited to worsen cholesterol crystal embolization.89
The timing of the initiation of warfarin anticoagulation after embolic stroke remains controversial. Embolic brain infarcts often become hemorrhagic, and serious brain hemorrhage has occurred after anticoagulation.90-94 Large infarcts, hypertension, large bolus doses of heparin, and excessive anticoagulation have been associated with hemorrhage. Because most hemorrhagic transformations occur within 48 hours, the recommendations of the Cerebral Embolism Task Force were to avoid early anticoagulation in patients with large infarcts or hemorrhagic transformation on repeat CT.95,96 Studies of patients with cerebral and cerebellar hemorrhagic infarction show that, in the vast majority, the cause is embolic, hemorrhagic infarction occurs equally with and without anticoagulation, and the development of hemorrhagic infarction is rarely accompanied by clinical worsening.97,98 Patients with hemorrhagic transformation who were continued on anticoagulants did not worsen. The risk of re-embolism must be balanced against the small but definite risk of important bleeding. However, if the patient has a large brain infarct, heparin should be delayed, and bolus heparin infusions should be avoided. If the risk for re-embolism is high, immediate heparinization is advisable; whereas if the risk seems low, it is prudent to delay anticoagulants for at least 48 hours. One study showed that patients with AF with embolic strokes who were treated with well-controlled heparin anticoagulation soon after stroke onset fared better than did patients treated later.99,100
Although once considered rare, emboli entering the systemic circulation through right-to-left shunting of blood are now often recognized with the advent of newer diagnostic technologies. By far the most common potential intracardiac shunt is a residual patent foramen ovale (PFO). The high frequency of PFOs in the normal adult population has made it difficult to be certain in an individual stroke patient with a PFO whether paradoxical embolism through the PFO was the cause of the stroke or whether the PFO was merely an incidental finding. Autopsy series have shown that approximately 30% of adults have a probe PFO at necropsy.101 Hagen and colleagues101 studied 956 patients with clinically and pathologically normal hearts and found a PFO in 27.35. The frequency of PFOs declined with age: 34.3% during the first 3 decades of life, 25.4% during the fourth to eighth decades, and 20.2% during the ninth and tenth decades. The average diameter of PFOs was 4.9 mm, and the size tended to increase with age.101 Echocardiographic studies have shown that PFOs are more common in patients with an undetermined cause of stroke than in those in whom another etiology has been defined.102-104 Lechat and coworkers,102 using TTE with contrast injection during Valsalva maneuver, demonstrated right-to-left shunting through a PFO in 56% of patients with cryptogenic stroke, in comparison to 10% of the patients in the control group. Webster and colleagues,105 in a study of stroke patients younger than 40 years of age, found a PFO in 50% of patients with stroke using contrast echocardiography. Di Tullio and coworkers103 demonstrated the presence of a PFO in 42% of patients with a cryptogenic stroke, compared with 7% in those with a determined etiology of stroke. This was observed in the younger (47% vs 4%) and the older (38% vs 8%) age subgroups.103
Neuroimaging studies are not conclusive with regard to the link between PFO and embolic stroke. However, in 1998, Steiner and colleagues106 reported on a series of 95 patients with first stroke and who had PFOs. Those with large PFOs had more features of embolic strokes with brain imaging than did patients with small PFOs.
Review of series of patients with paradoxical embolism107-109 through a PFO and the authors’ experience allow the derivation of five criteria that, when four or more are met, establish the presence of paradoxical embolism with a high degree of certainty6:
A situation that promotes thrombosis of leg or pelvic veins (eg, long sitting in one position, such as prolonged airplane flight, or recent surgery)
Increased coagulability (eg, the use of oral contraceptives, presence of factor V Leiden, dehydration)
The sudden onset of stroke during sexual intercourse, straining at stool, or other activity that includes a Valsalva maneuver or that promotes right-to-left shunting of blood
Pulmonary embolism within a short time before or after the neurologic ischemic event
The absence of other putative causes of stroke after thorough evaluation
Current treatment options for future stroke prevention in patients with PFO and ischemic stroke include medical therapy, open or minimally invasive cardiac surgical closure, and transcatheter closure. Before any treatment option is selected, it is important to confirm that the stroke is indeed cryptogenic. The treating physician should exclude all other possible contributing causes to the stroke, including a coexisting hypercoagulable state and deep venous thrombosis (with or without May-Thurner syndrome).110,111 Once this has been done, with regard to medical therapies, antiplatelet therapy is reasonable for future stroke prevention in cryptogenic stroke patients with a first ischemic stroke/TIA plus an isolated PFO. In patients with a cryptogenic stroke and an atrial septal aneurysm, evidence is insufficient to determine whether warfarin or aspirin is superior in preventing recurrent stroke or death, but minor bleeding is more frequent with warfarin.112 Warfarin is considered to be an appropriate treatment option in the subgroup of PFO/ischemic stroke patients with concomitant hypercoagulable state or venous thrombosis.
Transcatheter closure may ultimately demonstrate a benefit to medical therapy for future stroke prevention. A recent review of 10 nonrandomized unblended transcatheter closure studies for secondary stroke prevention reported a 1-year rate of recurrent neurologic events of 0% to 4.9% in transcatheter closure patients versus 3.8% to 12.0% in medically treated patients. The incidence of minor and major procedural complications was 7.9% and 1.45%, respectively. Other randomized trials evaluating the efficacy of transcatheter closure devices are ongoing.8,113 Small, separate trials have also suggested that, in highly symptomatic migraineurs with a history of PFO and stroke, transcatheter closure can also result in improvement of migraine severity in a high percentage of patients.114,115 At the time of this publication, transcatheter closure is not FDA approved for use after a first ischemic stroke in PFO patients; however, in PFO patients who fail medical therapy and have a second ischemic cerebral event, it is a treatment option to consider.
With regard to surgical closure of symptomatic PFO, there is no clear evidence at present that it is superior to medical or endovascular therapy for secondary stroke prevention. More recently, widespread use of intraoperative TEE during cardiac surgery has resulted in frequent discoveries of incidental asymptomatic PFOs. A recent survey by Sukernik et al116 suggests that a number of cardiac surgeons in the United States alter their planned procedure to include closure of the PFO. This is concerning in light of recent data published by Krasuski et al,117 who investigated the prevalence of intraoperative PFO diagnosis and the relationship that the incidental repair had on perioperative outcome and long-term survival. Surgeons were more likely to repair PFOs in patients who were younger, female, undergoing tricuspid or mitral surgery, had left atrial dilation, or with prior stroke and TIAs. Repair also tended to occur in patients with fewer comorbidities. Patients with incidental PFO were no more likely to have had a preprocedural stroke than patients without PFO. However, postoperatively, the patients with an incidentally repaired PFO had 2.47 times greater odds of having a postoperative stroke compared with those with unrepaired PFO (there was no difference in long-term survival).117 These findings of increased short-term postoperative stroke risk should discourage the routine closure of incidentally detected PFO for now. Further studies to assess whether any subgroup of PFO patients may benefit from closure will be important in the future.
After cardiopulmonary resuscitation (CPR), the heart often recovers in individuals whose brain has been irreversibly damaged by ischemic-anoxic damage.118 Cardiologists must become very familiar with the pathology, signs, and prognosis of brain dysfunction after periods of circulatory failure.
Different brain regions have selective vulnerability to hypoxic-ischemic damage. Regions that are most remote and at the edges of major vascular supply are more liable to sustain hypoperfusion injury. These zones are usually referred to as border zones or watersheds. The cerebral cortex and hippocampus are particularly vulnerable to injury.119-122 In the cerebral cortex, the border zone regions are between the anterior cerebral artery (ACA) and MCA and between the MCA and posterior cerebral artery (PCA). The basal ganglia and thalamus are most involved if hypoxia is severe but some circulation is preserved. This situation applies most to hanging, strangulation, drowning, and carbon monoxide exposure.123 Cerebellar neurons may also be selectively injured.124
When circulatory arrest is complete and abrupt, brainstem nuclei are especially vulnerable to necrosis in young humans and experimental animals.125 When hypoxia and ischemia are especially severe, the spinal cord may also be damaged.126,127 When cortical damage is severe and protracted, cytotoxic edema causes massive brain swelling, with cessation of blood flow and brain death.
Very severe hypoxic-ischemic damage can lead to mortal injury to the cortex and brainstem, irreversible coma, and brain death. When initially examined, such patients have no brainstem reflexes and no response to stimuli except perhaps a decerebration response. These findings do not improve, and respiratory control is absent or lost.
When cerebral cortical damage is very severe but brainstem reflexes are preserved, there is no meaningful response to the environment. Automatic facial movements such as blinking, tongue protrusion, and yawning usually persist. The eyes may rest slightly up and move from side to side. When this state does not improve, it is referred to as the persistent vegetative state118,121,128,129 or wakefulness without awareness. Laminar cortical necrosis can cause seizures (multifocal myoclonic twitches or jerks of the facial and limb muscles), which are difficult to control with anticonvulsants.
With severe hypoperfusion ACA-MCA border zone injury, there is weakness of the arms and proximal lower extremities with preservation of face, leg, and foot movement (the “man in a barrel” syndrome). With MCA-PCA ischemia, the symptoms and signs are predominantly visual. Patients describe difficulty seeing and inability to integrate the features of large objects or scenes despite retained capacity to see small objects in some parts of their visual fields. Reading is impossible. There are features of Balint syndrome.118,130 Apathy, inertia, and amnesia are also common. Patients cannot make new memories and have patchy, retrograde amnesia for events during and before hospitalization. This Korsakoff-type syndrome is caused by hippocampal damage and may not be fully reversible. Amnesia may be accompanied by visual abnormalities, apathy, and confusion, or may be isolated.
Shortly after resuscitation or arrest, patients with less severe cerebral injuries show some reactivity to the environment. Eye opening and restless limb movements develop. The eyes may fixate on objects. Noise, a flashlight, or a gentle pinch may arouse patients to react to stimuli. Soon patients awaken fully and may begin to speak. Cognitive and behavioral abnormalities may be detected after the patient awakens, depending on the degree of injury.
Prognostic signs and variables have been extensively studied.118,131-134 The initial neurologic findings and their course are helpful in predicting outcome. Among patients who have meaningful responses to pain at 1 hour, almost all survivors have preserved intellectual function. Patients who do not respond to pain by 24 hours typically either die or remain in a vegetative state. Being comatose predicts a poor prognosis.133,134Thus, two simple observations—the presence or absence of coma and the response to pain—predict neurologic outcome very early.134 Recurrent myoclonus is also a poor prognostic sign. 135
In a study in Seattle of out-of-hospital cardiac arrests, patients who did not awaken died on average 3.5 days after arrest.136,137 Of 459 patients, 183 never awakened (40%). Among those who did awaken, 91 (33%) had persistent neurologic deficits.136 Prognosis could be made by analysis of pupillary light reflexes, eye movements, and motor responses.137 It is unclear whether bystander initiation of CPR is significantly related to awakening.137,138 After in-hospital CPR, pneumonia, hypotension, renal failure, cancer, and a housebound state before hospitalization were significantly related to death in the hospital.139
Neuroimaging and other tests have proved to be relatively unhelpful in contrast to the neurologic examination.118 CT is used to exclude other causes of coma such as brain hemorrhage. Electroencephalography is helpful in studying cortical activity in unresponsive patients. TCD may be helpful in the evaluation of brain death.140-142
Other than maintaining adequate circulation and oxygenation, treatment has not helped improve outcome. Increased blood sugar correlates with poor outcome.143 A multifaceted approach to therapy has been most successful.144
Drugs given to patients with cardiac disease often have neurologic adverse effects.145 Digitalis can cause visual hallucinations, yellow vision, and general confusion.146,147 Digitalis levels need not be excessively elevated; the symptoms disappear with drug cessation. Quinidine can cause delirium, seizures, coma, vertigo, tinnitus, and visual blurring.148 Similar toxicity has been seen with lithium. Patients may become acutely comatose while being treated with intravenous lidocaine. This effect has been associated with the accidental administration of very large doses; more common CNS effects of less extreme toxicity include sedation, irritability, and twitching. The latter may progress to seizures accompanied by respiratory depression. Amiodarone can cause ataxia, weakness, tremors, paresthesias, visual symptoms, a Parkinsonian-like syndrome, and occasionally delirium.145
Patients with congestive heart failure often develop an encephalopathy characterized by decreased alertness, sleepiness, decrease in all intellectual functions, asterixis, and variability of alertness and cognitive functions from hour to hour.145 These patients may not have pulmonary, liver, or renal failure or electrolyte abnormalities. This cardiac encephalopathy is probably multifactoral.145
Neurologic and Cerebrovascular Complications of Endovascular Cardiac Procedures and Cardiac Surgery
Patients with heart disease are diagnosed, treated, and at times even cured with a variety of cardiac procedures. Although the implicit goal with any cardiac intervention (diagnostic or therapeutic) is to improve a patient’s quality of life, these procedures often carry risk as well as the possibility of benefit.
Stroke and TIA are known complications of heart catheterization. In 1977, Dawson and Fischer149 reported cerebrovascular complications in 10 of 1000 consecutive cardiac catheterizations. Nine out of 10 of these events were determined to be embolic.149,150 Similarly, Mendez Dominguez and colleagues151 reported thromboembolic neurologic complications in seven patients in a series of 2178 consecutive cardiac catheterizations. In all cases, the cerebrovascular impairment occurred either during or within several minutes following the cardiac catheterization. All strokes were confirmed with CT or MRI, and the clinical profile in most cases supported an embolic mechanism.151 Central retinal artery occlusion has been reported in association with cardiac catheterization.152 More recently, Liu and coworkers153 reported six ischemic strokes and one intracerebral hemorrhage in a series of 3648 cardiac catheterizations in children. In this study, the suspected catheterization-related stroke mechanisms included intracranial hemorrhage caused by intraprocedure anticoagulation as well as cerebral embolism from local clot.153 Other potential mechanisms for cerebrovascular events during cardiac angiography may include catheter tip thromboembolism, atherosclerotic plaque or cholesterol embolism, air emboli, arterial vasospasm, and/or hypotension.150,154-157
The stroke rate in patients undergoing percutaneous coronary interventions for both stable and unstable coronary artery disease (including angioplasty for acute MI) has been reported to be between 0% and 4%.158-165 A combined analysis of data from four double-blind, placebo-controlled, randomized trials (EPIC, CAPTURE, EPILOG, and EPISTENT) conducted between 1991 and 1997 assessed 8555 patients undergoing various percutaneous coronary interventions. Among the 8555 patients, there were 33 strokes in 31 patients (0.36%) within 30 days. Stroke occurred in 9 (0.29%) of 3079 patients receiving percutaneous coronary interventions alone; six strokes were ischemic, and three were hemorrhagic. Stroke was diagnosed in 22 (0.41%) of 5476 patients who underwent percutaneous treatment in conjunction with abciximab treatment, of which there were 13 ischemic strokes and 9 hemorrhages.158-163
Galbreath and colleagues164 reported a 0.2% rate of focal central neurologic complication in their series of 1968 percutaneous transluminal coronary angioplasties; three were ischemic strokes, and one was a TIA. The mechanism in these cases was determined to be embolic in three cases; one patient had air inadvertently injected through the guide catheter, and two had events after the ascending aorta was scraped with the guide catheter in search of a graft ostium. The remaining patient had an event during a period of hypotension.164
Thromboembolic stroke can be a complication of cardiac electrophysiologic procedures, including radiofrequency catheter ablation of arrhythmia. Multicenter data are limited; however, the stroke risk appears to be <2%.150,166-169 Additionally, electrical cardioversion may be used in the treatment of AF and atrial flutter. Stroke caused by direct current cardioversion has been estimated to occur in 1.3% of cardioverted patients.170 Anticoagulation before and after cardioversion lowers the risk of embolism.150,170,171
In patients with nonvalvular AF, embolic stroke is thought to be associated with left atrial appendage (LAA) thrombi. One multicenter, randomized, noninferiority trial found that percutaneous closure of the LAA was noninferior to warfarin treatment. In the future, closure of the LAA might provide an alternative to chronic warfarin for stroke prevention in patients with nonvalvular AF.172 A second study investigated percutaneous closure of the LAA in patients who were not warfarin candidates. At the 5-year follow-up, the annualized stroke/TIA rate was 3.8% per year, which was lower than predicted.173
Surgical treatment, specifically valve replacement, is still the definitive treatment of choice for both aortic and mitral valve diseases, rather than percutaneous valvuloplasty. Aortic valvuloplasty, at present, provides only transient and modest benefit for aortic stenosis, with a significant risk of stroke and vascular injury. However, it can stabilize patients who require additional attention prior to undergoing surgery.174 Additionally, over the last 5 years, percutaneous approaches to valve implantation have improved. Technical and device issues are being refined, and some percutaneous treatments are showing promise in ongoing clinical trials.175 Percutaneous balloon aortic and mitral valvuloplasties have been complicated by stroke.150,176-179 Sudden coma has also been reported after percutaneous balloon mitral valvuloplasty.180 With regard to when the neurologic events occur, the 1988 series of Letac and coworkers176 of 218 patients undergoing transcutaneous balloon aortic valvuloplasty indicates that one stroke occurred during the intraprocedure period, whereas three additional strokes occurred during the postprocedure period.150,176-179
Intra-aortic balloon pumps (IABPs) are used in patients with severe left ventricular failure or cardiogenic shock. The IABP is inserted into the patient’s midthoracic aorta to maintain adequate perfusion. Spinal cord infarcts can occur in patients with IABPs caused by local thromboembolism, aortic dissection, aortic atherosclerotic plaque rupture, or local hypoperfusion.150,181
Every year, an estimated 1 million patients undergo cardiac surgery throughout the world. Coronary artery bypass graft (CABG) surgery is the most common major cardiovascular operation performed.
The frequency of abnormalities of intellectual function and behavior after cardiac surgery is high.182,183 Preoperative diagnoses of diabetes, history of prior stroke, older age, female sex, smoking, hypertension, left main coronary disease, mild renal impairment (defined as serum creatinine 1.47-2.25 mg/dL), and high-sensitivity preoperative C-reactive protein (defined as high-sensitivity C-reactive protein concentration ≥3.3 mg/L) have all been identified to increase perioperative stroke risk.184-188 Additionally, preoperative stroke and TIA are also risk factors for in-hospital mortality.187 The cerebrovascular risk depends on the particular procedure performed. Estimations of stroke risk for isolated CABG range from 0.8% to 3.8%.189-193 A particular patient’s risk of perioperative stroke may be estimated using the Society of Thoracic Surgeons 2008 cardiac surgery risk models, which include outcomes for stroke, as well as deep sternal wound infection, reoperation, prolonged ventilation, and renal failure, among other morbidities. Several variables were forced into each model to ensure face validity (eg, the permanent stroke model includes AF as a variable).194-196
The majority of studies presented in current literature suggest that there is a decrease in postoperative stroke rates in patients undergoing off-pump CABG compared with patients undergoing the traditional on-pump operation, but conflicts in the literature do exist.197-205 One potential explanation for the discrepancy is that the temporal pattern of stroke after CABG was not distinguished in all studies. One single-center study of 2516 consecutive patients has noted that off-pump CABG reduced the incidence of early postoperative stroke (symptoms noted just after emergence of anesthesia). However, the risk of delayed stroke (normal neurologically emerging from anesthesia, but symptoms presenting within 30 days after surgery) was no different between the on- and off-pump CABG patients.205 Perhaps the difference in the early stroke etiology between off- and on-pump CABG could be explained by the difference in clamp use between these CABG procedures.
Another reason for the discrepancies in data may be that many of the above studies also did not differentiate between clampless and partial clamp off-pump techniques. One study of 700 consecutive patients undergoing multiple-vessel off-pump CABG demonstrated a decreased incidence of stroke (0.2% vs 2.2%) in the aortic no-touch group. Additionally, logistic regression identified partial aortic clamping as the only independent predictor of stroke, increasing this risk 28-fold.206 Interestingly (and perhaps also due to the difference in clamp use between on- and off-pump CABG procedures), one study also found that off-pump CABG provided a decreased risk of stroke and mortality advantages, especially for women.204
In one multicenter study, the incidence of stroke plus severe intellectual dysfunction from CABG has been reported to be 6%.207 With regard to combined procedures, one multicenter investigation assessing 273 patients undergoing combined CABG and left-sided cardiac procedure (such as aortic or mitral valve replacement) estimated that 15.8% of patients had neurologic complications (8.5% with stroke or TIA and 7.3% with new intellectual deterioration).208 This combined procedure appears to carry a higher stroke risk than CABG performed in isolation.
It is unclear whether minimally invasive cardiac surgical procedures may potentially have a lessened stroke risk because further data are needed. One study suggests that minimally invasive CABG may lessen the risk of major adverse cerebrovascular and cardiac events compared with traditional off-pump CABG.209 Another center’s result with direct-access, minimally invasive mitral valve surgery in 106 patients demonstrated a low rate of stroke and TIA, with a total of 0.28% of patients having either stroke or TIA. However, a second center’s results with minimally invasive, reoperative, isolated valve surgery did not show a lower stroke rate compared with patients undergoing sternotomy.210,211
Overall, in prospective studies, transient complications have been noted in 61% of cardiac surgery patients.212 In one series in CABG patients, 16.8% had stroke or encephalopathy postoperatively; the encephalopathies usually cleared, and only 2% of patients had severe strokes.213 The potential mechanisms of cerebral impairment in the cardiac surgery population will be explored in the following sections.
An estimated 12% of patients requiring CABG also have significant carotid artery disease.211 One major concern regarding cardiac surgery patients has been whether the hemodynamic stress of heart surgery leads to underperfusion of areas supplied by already stenotic or occluded arteries, resulting in brain infarcts. This concern underlies neck auscultation for bruits, ultrasound carotid artery testing, and cerebral angiography prior to CABG. However, hemodynamically induced infarction related to preexisting atherosclerotic occlusive cervicocranial arterial disease is a rare complication of heart surgery. Asymptomatic patients with carotid bruits have a very low rate of stroke after elective surgery.214,215 However, the risk of perioperative stroke does increase with increasing severity of carotid stenosis.190,216,217 In a retrospective study of CABG patients with known carotid disease, ipsilateral strokes occurred in 1.1% of arteries with 50% to 90% stenosis, in 6.2% of arteries with >90% stenosis, and in only 2% of vessels with carotid occlusion.216,217
Uncertainty exists about whether carotid endarterectomy (CEA) performed simultaneous or prior to CABG improves perioperative stroke risk. Stroke rates vary greatly in those undergoing combined CEA-CABG as opposed to staged procedures.218 Limited published data of 324 patients suggested that early outcomes after synchronous CEA plus off-pump CABG were better than those following staged or synchronous CEA plus on-pump CABG. It is unclear whether these findings were due to publication bias, case selection, or, as we discussed earlier in the chapter, the fact that the aorta was not manipulated or cannulated.219
Definitive management of combined cervicocerebral and coronary artery disease awaits the outcome of clinical trials. One systematic review of 97 published studies of staged versus synchronous operations found no significant difference in outcomes between these two surgical groups. Unfortunately in this study, there were no comparable data for patients with combined carotid stenosis and cardiac disease not undergoing either staged or synchronous surgery.220 A different retrospective study of 4335 patients undergoing cardiac surgery found that undergoing a combined CEA-CABG increased the risk of postoperative stroke compared with patients with similar degrees of carotid stenosis who underwent CABG without a carotid revascularization.221
Carotid artery stenting (CAS) has been recently introduced as an alternative revascularization modality in high-risk patients. Although some studies suggest that endovascular CAS for both symptomatic and asymptomatic carotid artery disease prior to CABG is safe and without an increased risk of stroke, conflicts again arise in the literature.222-226 Whether CAS should be performed prior to CABG is still a point of debate at this time.
Most studies have relied on clinical localization of focal deficits and inference about their mechanisms. A neuroradiology study reviewed neuroimaging results from 30 patients with acute strokes in relation to CABG.227 Only one had evidence of a hemodynamic atherostenotic mechanism, which supports the data suggesting that hemodynamically induced infarction during cardiac surgery is rare. Embolism arising from cardiac and aortic sources is much more common than atherothrombotic infarcts and is of a much greater concern.182
One point against a hemodynamic or hypoperfusion cause of many strokes is their timing. It appears that strokes may occur more frequently after recovery from the anesthetic.205,228,229 If the mechanism of stroke were hemodynamic, the major circulatory stress would be intraoperative and patients would at least awaken from anesthesia with the deficit. In two studies in which the authors record the timing of coronary artery bypass surgery–related strokes, only 16%228 and 17%229 of patients had deficits noted immediately postoperatively. The distribution of infarcts and their multiplicity on neuroimaging scans were most consistent with embolism. Embolic infarcts may involve either the anterior or the posterior circulation.182,213,227,228 In one series of postoperative, posterior circulation strokes, most were embolic and followed cardiac surgery.229
In cardiac surgery patients, the preponderance of evidence suggests that macroemboli (>200 μm in diameter) and microemboli are responsible for most neurologic complications.207,213,220,230,231 Macroemboli (associated with atherosclerotic plaque disruption or rupture) are believed to precipitate focal deficits, whereas particulate microemboli (white blood cell and platelet aggregates, fat, or air) may be implicated in more subtle diffuse cognitive dysfunction.207,232
Emboli may arise from preexisting cardiac abnormalities (such as hypofunctioning ventricles, dilated atria, and aortic atheromas) or from postoperative arrhythmias.172 Evidence links operative and postoperative embolism to aortic ulcerative atherosclerotic lesions. Cross-clamping of the ascending aorta and aortotomy liberate cholesterol or calcific plaque debris.182,233 Data from a series of 2641 consecutive cardiac surgery patients showed that left-body symptoms (right hemispheric stroke) were twice as common as right-body symptoms, suggesting that aortic manipulation and anatomic mechanisms in the aortic arch were more likely to cause cerebrovascular accidents than effects from aortic cannula stream jets.234 Figure 107–1 shows the aorta of a patient who died having never awakened after CABG. Figure 107–2 shows cholesterol crystals and other debris trapped within a filter placed in the aorta at the time of unclamping.
Given that atherosclerosis of the ascending aorta is a risk for perioperative stroke,235 it was postulated that avoiding direct manipulation of this area may improve neurologic outcome postoperatively. Epiaortic ultrasound scanning is thought to be superior to both manual palpation of the ascending aorta and TEE in detecting atherosclerosis, particularly noncalcified plaque. It has led to modifications in surgical management in patients undergoing CABG, such as modification of cannulation, clamping, or anastomotic technique, and temperature management.236-238 One study suggested that the application of aortic clamping or cardiopulmonary bypass was not a risk when the ascending aorta was evaluated using epiaortic ultrasound.236 A second study of 6051 patients also noted that the overall stroke risk was lower in patients who had intraoperative epiaortic ultrasound, compared with all patients undergoing cardiac surgical procedures.239 However, why the stroke risk is lower is not yet clear; a separate study found that the use of this imaging technique did not lead to a reduced number of TCD ultrasound–detected cerebral microemboli before or during bypass.237 One might theorize that this technique reduces macroemboli and not microemboli.
In another series in which embolic signals were monitored during CABG surgery, 34% of signals were detected as the aortic cross clamps were removed, and another 24% were detected as aortic partial occlusion clamps were removed.233 The number of microemboli detected correlated with abnormalities of cognitive function after surgery.182,240 Figure 107–3 shows microemboli within the aorta shown by TEE after release of aortic clamps. Figures 107–4 and 107–5 are TCD recordings during manipulation of the aorta and after release of aortic clamps.
In a similar study within a Chinese population of 227 patients, off-pump CABG surgery was noted to significantly decrease the number of cerebral microembolic events detected by TCD. This also could be explained by the aortic clamping technique required for traditional CABG. Interestingly, though, this study also found that the incidence of postoperative cognitive dysfunction was not decreased by off-pump CABG, which suggests that microemboli may not be the responsible culprit for this phenomenon.241
Thromboembolic infarction often occurs in the days following surgery when cessation of anticoagulation is necessary. Postoperative activation of coagulation factors in cardiac surgery patients can promote hypercoagulability. Disseminated intravascular coagulation, acquired antithrombin III deficiency, and acquired protein C deficiencies are not uncommon. Activation of the coagulation-fibrinolytic system can persist for 2 months after cardiopulmonary bypass surgery.242,243 In some patients, hypercoagulability related to surgery can precipitate occlusive thrombosis in atherostenotic arteries, and the newly formed thrombus can lead to intra-arterial embolism. Cardiac, aortic, and intra-arterial embolism accounts for the vast majority of cardiac surgery–related focal neurologic deficits.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree