Cerebral oxygen delivery (CDO2) and cerebral oxygen consumption (CMRO2) versus mean arterial pressure (MAP) during cardiopulmonary bypass (CPB) at 33°C. Values for oxygen delivery (on ordinate in ml/100 g per minute) are the mean ± standard deviation (*P < 0.05 versus MAP of 60 mmHg by repeated-measure analysis of variance followed by Student-Neuman-Keuls test)
After MAP and HCT, cerebral metabolism is a primary determinant of cerebral blood flow. Over the temperature range in which most adult bypass is conducted, 27°C to 37°C, there is a clear relationship between temperature, CMRO2, and CBF. Below 25°C, the relationships become much more complex. If all other variables (primarily MAP, HCT, and CO2) are controlled, a 10°C decrease in temperature reduces CMRO2 by about 60% and this is associated with a 50% reduction in CBF.
PaCO2 is an independent determinant of CBF during bypass. However, during most adult cardiac surgery the effect of PaCO2 is relatively small. If all other variables are controlled, every 1 torr increase or decrease in PaCO2 alters CBF approximately 3%. As such, between 32°C and 37°C the maximal effect of CO2 on CBF is about 15%. The effect of CO2 and alpha-stat/pH-stat strategies becomes increasingly relevant below 27°C, but it is a minor consideration above 32°C.
Cerebral physiology during CPB has been somewhat difficult to determine because so many variables are subject to change simultaneously and some of the physiological variables interact. This is particularly notable in the interactions of HCT, MAP, and CBF. The interaction of variables leads to confusion about the effect of pump flow on CBF. Some literature has reported that cerebral perfusion is dependent on pump flow. This misunderstanding arose from the failure to appreciate that pump flow, like cardiac output, is a primary determinant of mean arterial pressure. While decreases or increases in CBF may be seen when pump flow is increased or decreased, this is really only clearly demonstrated below or near the autoregulatory threshold: above a MAP of approximately 55 mmHg, increases in pump flow do not increase CBF, while below about 55 mmHg reductions in pump flow result in reductions in MAP that in course lead to reductions in CBF. This was well demonstrated in an animal study by Sadahiro (see Figure 12.2).
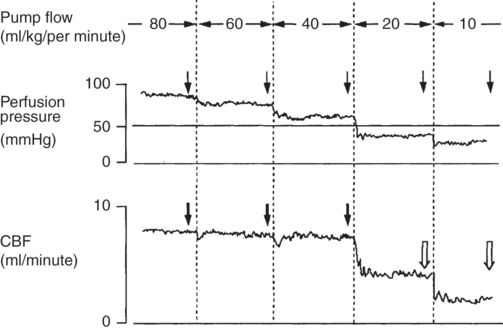
Continuous monitoring of perfusion pressure and CBF during perfusion flow rates from 80 to 10 ml/kg/minute. Arrows indicate the point at which the relationship between CBF and perfusion pressure was evaluated. Black arrows show the presence of an autoregulatory response with CBF returning to its prior level after an initial drop. White arrows show the loss of a vascular response
The dependence of CBF on pump flow is seen only when pump flow is too low to generate a MAP above the autoregulatory threshold. In humans and in animals, the independence of CBF from pump flow between 1.2 and 2.3 l/minute/m2 at a stable MAP has been well shown at 27°C (see Table 12.1).
| 30 adult patients, CPB at 27°C | High flow | Low flow |
|---|---|---|
| Pump flow (l/minute/m2) | 2.3 ± 0.1 | 1.2 ± 0.1 |
| MAP (mmHg) | 63 ± 9 | 62 ± 6 |
| CBF (ml/100 g/minute) | 29 ± 7 | 30 ± 8 |
Pulsatile flow appears to have no effect on cerebral blood flow during CPB independent of any effect of pulsatility on MAP.
Intraoperative ischemia and physiological management
Of the strokes caused in the operating room, watershed infarcts constitute the minority of cerebral ischemic events. When available, neuroimaging usually demonstrates embolic events and associated regional hypoperfusion. This is probably why, in spite of intensive clinical and laboratory study, it has been difficult to show that physiological variables, such as temperature or perfusion pressure, during CPB are independent determinants of neurological outcome. For intraoperative strokes it is more likely that these variables modulate the severity of injury that occurs subsequent to a cerebral embolic event. Because of the frequency of cerebral ischemic events, physiological management remains a relevant part of practice even if it does not prevent most strokes.
Effect of perfusion pressure
Through much of the 1980s the surgical, and some of the anesthesia literature, indicated that the cerebral autoregulatory threshold was shifted leftward during bypass such that lower mean arterial pressures (35–40 mmHg) were capable of maintaining normal cerebral blood flow. This was incorrect. Although hemodilution associated with CPB increases cerebral blood flow for any given mean arterial pressure, the autoregulatory curve still “breaks” at a pressure of approximately 55 mmHg; below this level cerebral blood flow is compromised. A combination of well-conducted laboratory investigations, the clinical investigation by Gold and colleagues showing better composite cardiac and neurological outcomes at higher mean arterial pressures, and a better understanding of cerebral autoregulation in diabetic and hypertensive patients as well as the elderly have led clinical practice to maintain MAPs above 55–60 mmHg during CPB. Rather than preventing watershed infarcts, this practice presumably helps to maintain cerebral perfusion in the presence of carotid, cerebral, and penetrating vessel disease and supports collateral flow and perfusion of the peri-ischemic region when embolic events do occur.
Effect of temperature
Given the profound effects of temperature on cerebral oxygen demand and the widely held belief in the neuroprotective effect of hypothermia, it was reasonable to expect that absolute CPB temperature would be identified as a primary determinant of cognitive outcome. However, this has not been the case in randomized or non-randomized trials. Although there are “multiple” publications investigating the effect of perioperative temperature management on neurological outcomes, the weight of the evidence is far weaker than would be expected. This is not to say that perioperative temperature management is unimportant, only that the best evidence of an effect of a hypothermic management on neurological outcome is quite weak. While the debate over the relative benefits of hypothermic versus normothermic bypass remains unresolved, more detailed examination of the influence of re-warming rate and postoperative temperature in determining cognitive outcomes have produced interesting results.
Data from the 2001 Grigore’s study randomizing patients to different intraoperative temperature management regimens was reported again when a subset of that negative outcome trial was re-analyzed taking into account the effect of the rate of re-warming on cognitive outcomes. In this re-analysis slow re-warming was associated with a beneficial effect on cognitive performance compared to conventional re-warming.
The simplest improvement in clinical practice relating to temperature management during CPB followed the first documentation of cerebral hyperthermia in 1996. Cook et al. showed that brain temperature was systematically underestimated during CPB and that cerebral temperature can approach 40°C during re-warming, a period associated with a great number of embolic events. From this observation, closer monitoring of nasopharyngeal and perfusate temperature and prevention of hyperthermia during CPB have become a standard part of intraoperative care.
Effect of glucose control
Maintaining blood glucose in the normal range is more a matter of not doing harm than actually doing something to prevent or reverse ischemic injury. The experimental stroke literature clearly demonstrates that hyperglycemia, like hyperthermia, worsens neurological outcome in the event of an ischemic insult. While maintaining perioperative normoglycemia will not independently determine the incidence of perioperative stroke, it is very likely to moderate its severity when ischemia does occur.
Effect of other measures
Few other intraoperative interventions hypothesized to improve neurological outcome have found their way into clinical practice. A range of different classes of drugs have been tried including aprotinin, complement inhibitors, steroids, barbiturates, propofol, xenon, calcium channel antagonists, and magnesium, to name but a few. None has proved efficacious in a sufficiently powered clinical trial.
Perioperative stroke
Stroke is one of the most devastating complications following adult cardiac surgery. Literature from the 1960s and 1970s indicates that the incidence of stroke was approximately 2–4%. When one looks at very large populations, reports from the last 5 years demonstrate that this is largely unchanged. The overwhelming risk factor for stroke is physiological age, particularly manifest as atherosclerotic disease. In the 1990s a great deal of attention was paid to whether physiological variables were responsible for neurological outcomes, but none of these studies produced compelling evidence. With the rapid expansion of echocardiography and transcranial Doppler studies, attention shifted towards intraoperative embolization as the primary etiology of perioperative stroke and cognitive dysfunction.
While difficult to measure, due to lack of a concurrent control group, improvements in surgical technique have probably restricted the rise in the incidence of stroke, which would have been anticipated in the ageing population of patients, with more complex medical conditions, presenting for cardiac surgery. Thus, even if the overall stroke incidence in cardiac surgery is relatively unchanged, at least it has not risen to the levels that outcome models would predict. Recognition of the importance of embolic stroke has led to increased care in handling of the ascending aorta. Transcranial Doppler, echocardiographic, and neuroimaging data all point to the ascending aorta as the primary source of emboli leading to intraoperative stroke. Measures that probably reduce intraoperative embolization include intraoperative imaging of the ascending aorta to assess the optimal site (i.e. area with minimal atherosclerosis) for cross-clamp application, single application of the aortic clamp, femoral cannulation, all-arterial grafting, and off-pump techniques that eliminate ascending aorta instrumentation. However, even with excellent surgical management of the ascending aorta there still remains a substantial incidence of perioperative brain injury.
Use of Epiaortic scanning
Epiaortic scanning is a relatively low-cost intervention that may help to reduce the incidence of strokes due to embolization of atheromatous plaque displaced by surgical manipulation of the ascending aorta. Epiaortic scanning has been demonstrated to be more sensitive than digital palpation for identifying normal aorta for cannulation or cross-clamp application. Hangler and colleagues used epiaortic scanning to guide their management of the ascending aorta in 352 CABG patients and reduced their stroke rate from 4.4% to 2.9% when compared to historical data. In a study of 909 CABG patients by Yamaguchi and colleagues, the authors identified 196 patients for whom aortic manipulation might be unsafe and thus total arterial grafting using an off-pump technique was employed. The remaining 713 patients either had off-pump or traditional on-pump CABG and their aortic manipulation was guided by epiaortic scanning. No in-hospital strokes were identified in any patients, suggesting that risk of embolic stroke from aortic cannulation and cross-clamping is greatly reduced when epiaortic scanning is used. In cardiac operating rooms, equipment for epiaortic scanning is typically readily available given the widespread application of TEE. However, using epiaortic scanning to its full potential requires skill in the interpretation of the aortic ultrasound images, as well as surgeons willing to alter their approach to the ascending aorta when faced with severe atherosclerotic disease.
Off-pump CABG
Off-pump CABG (OPCABG) evolved from minimally invasive surgery and a desire to eliminate any morbidity associated with CPB. There was an expectation that elimination of CPB would dramatically reduce the number of perioperative strokes. Interestingly, this has not been fully borne out. Stroke rates vary greatly in cardiac surgical reports depending on the patient population and the type of surgery; however, in single institution CABG surgery, aortic “no-touch techniques” or off-pump surgery seem to only moderately reduce stroke risk. In a large-scale study of over 16 000 patients, Bucerius described a stroke rate of 3.9% in CABG with conventional bypass versus 2.5% in the off-pump group. A similar effect of off-pump surgery is identified by Peel and colleagues who, in a study population of almost 3300 off-pump and 7300 on-pump CABG, found a stroke rate of 1.35% in off-pump and 2.4% in on-pump CABG (see Figure 12.3). This effect of eliminating aortic cannulation, about a 1% decrease in stroke incidence, is similar to that described in a meta-analysis of off-pump surgery results, as well as the stroke reduction identified with surgical management guided by epiaortic scanning. This moderately small, but meaningful effect is important because it indicates that more than half of perioperative strokes may not be related to intraoperative embolization from the aorta. The limited neuroimaging data available support this: brain imaging shows a 30% incidence of subclinical cerebral ischemic events in OPCABG.
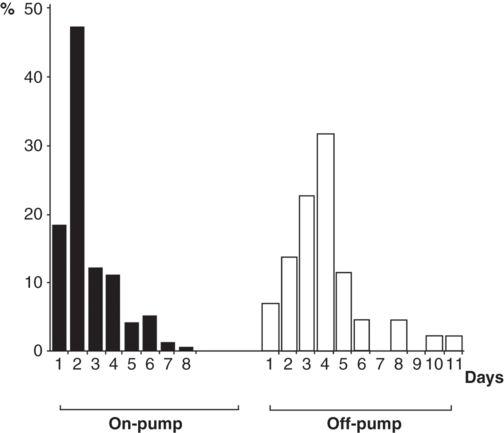
Chronological distribution of the onset of postoperative stroke for on-pump and off-pump CABG
Aortic “No-Touch” or total arterial grafting
Although avoiding cannulation of the ascending aorta is associated with a stroke reduction of approximately 1% in OPCABG, many OPCABG cases still involve a side-biting aortic cross-clamp, which has been implicated as a source for embolic debris. Newer OPCABG techniques using total arterial conduits with bilateral internal thoracic arterial conduits do not require proximal aortic anastomosis so are viewed as the most practical way of reducing embolization from aortic manipulation. While theoretically advantageous, this technique is often insufficient at providing conduits of adequate length to address all areas requiring revascularization. While graft extension with free grafts is helpful, supplementation with additional grafts (requiring proximal aortic anastomoses) is often required to achieve complete revascularization. Newer devices such as the HEARTSTRING proximal seal system (Guidant Corporation) allow formation of proximal aortic anastomosis without the application of aortic cross-clamping. A study published by Emmert and colleagues in 2011 compared 4314 patients undergoing either on-pump or OPCABG. The OPCABG group was further subdivided into patients that received a proximal side clamp or the HEARTSTRING system. It is unclear as to how the authors divided the OPCABG group into proximal side clamp or HEARTSTRING cohorts. Patients receiving total arterial grafting without aortic manipulation acted as a control. There was a net 1% stroke reduction when comparing the on-pump and OPCABG groups. However, the subgroup using the HEARTSTRING system had a 0.7% stroke rate vs 2.3% for the traditional side-biting clamp. The 0.7% stroke rate is similar to that seen with the total arterial graft group (0.8%). This data suggests that perhaps stroke reduction can be obtained during OPCABG using techniques that avoid side-biting clamps on the proximal aorta. While off-pump techniques or total arterial revascularization are not suitable for all patients or all surgeons, there is clear evidence that they are advantageous and should probably be considered whenever practical. There is also data to suggest that strokes that occur with off-pump and on-pump CABG have different timing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


