16
Central venous and dialysis access
Central venous access
In addition to central venous pressure monitoring, CVCs can be used to infuse large volumes of irritant solutions, such as antibiotics, blood products, parenteral nutrition and chemotherapeutic agents, particularly if required over long periods. In an emergency, CVCs allow the rapid administration of large volumes of fluid if peripheral access cannot be achieved. Other indications include haemodialysis and plasmapheresis. Implantable injection ports, ‘portacaths’ (e.g. Bardport, Passport, Infuse-a-Port or MediPort), may be used for chemotherapy or long-term administration of other drugs.1 One has also been developed for haemodialysis (Lifesite).2
Methods
CVCs are generally inserted under local anaesthetic through the internal jugular, subclavian or femoral veins, preferably using ultrasound guidance, by the Seldinger technique.3 If short-term access is required a multilumen catheter is inserted into the internal jugular vein so that the tip lies in the superior vena cava. For long-term access a catheter with an attached Dacron cuff is placed in a subcutaneous tunnel (e.g. Hickman line) for fixation and to act as a barrier to infection.
Temporary dialysis access
Renal replacement therapy may be accomplished by renal transplantation or peritoneal dialysis but most patients require at least a period of haemodialysis. About 75% of patients are known to have deteriorating renal function at least 90 days before dialysis is required so that permanent haemodialysis access can be created in advance. Unfortunately, this opportunity is frequently missed in UK practice, with less than a third of patients starting haemodialysis with definitive access.5 Dialysis is required for hyperkalaemia or when symptoms of weight loss, nausea, vomiting, anorexia or itching occur, usually at a serum creatinine level of 500–1500 mmol/L.
For patients presenting as an emergency with end-stage renal disease (ESRD) without prior access, haemodialysis can start using a double-lumen CVC whilst awaiting a permanent AVF. However, CVCs have a high risk of infection,6 cause central venous stenosis or thrombosis7 compromising further access in the upper limbs, and have a higher mortality than AVFs,8 so should not be used long term except where other options have been exhausted.
The subcutaneous tunnel may reduce the rate of infection, but this has not been proven in a randomised trial.9
Methods
Temporary femoral vein catheters are useful for acute dialysis but have a higher rate of infection than internal jugular CVCs10 and should be replaced by a tunnelled (preferably jugular) venous catheter at the earliest opportunity. A median survival of 166 days has been reported for tunnelled femoral CVCs.11
The catheter tip is usually placed at the SVC/RA junction. Atrial placement minimises recirculation and reduces the risk of migration on standing,12 but may cause arrhythmias.
Complications of CVCs
Catheter-locking solutions
Catheter patency can be maintained between dialysis sessions using a catheter-locking solution. The standard procedure has been heparin instillation (1000–10 000 u/mL) into the catheter lumen in a volume sufficient to fill to the lumen tip. There is a risk of heparin loss due to diffusion into the blood stream and unintentional systemic anticoagulation. Low-dose heparin (1000–2500 u/mL) seems as effective as high-dose.14 Trisodium citrate, which also has antibacterial properties, is also an effective catheter lock but there is no randomised trial versus heparin.15
A recent randomised study compared 225 patients on haemodialysis who had a central venous catheter using a locking regime of heparin 5000 u/mL or recombinant tissue plasminogen activator (rt-PA) 1 mg in each lumen. The rate of catheter malfunction in the heparin group (34.8%) was significantly worse than in the patients assigned to rt-PA and the risk of bacteraemia was three times higher than in the heparin group.16
Catheter lumen thrombosis
Catheter thrombosis is the most common cause of poor long-term function.17 Prophylactic warfarin can be effective at reducing thrombosis18 but there are no randomised data comparing international normalised ratio (INR) ranges and controls. There is a risk of bleeding and a need for regular monitoring.
Catheter malfunction due to thrombus can be treated by lytic agents such as rt-PA or urokinase.
Lytic agents are also used for the treatment of catheter thrombosis. An instillation of rt-PA 1 mg/mL for 30 minutes restored or maintained a flow rate of greater than 300 mL/min without line reversals in 36 of 50 (72%) patients, with a second instillation restoring patency for a further four patients (80%). The majority of patients required further thrombolysis or radiological intervention in the 4-month follow-up period.19
The optimal dwell times for lytic agents have yet to be determined. rt-PA infusions are effective even when there is an associated fibrin sheath.20
Tenecteplase is a new lytic agent with increased fibrin specificity, greater resistance to plasminogen activator inhibitor 1 and a relatively long half-life. A randomised study showed a 1-hour dwell of 2 mg of tenecteplase more effective than placebo in restoring flow in dysfunctional haemodialysis catheters.21 An extended dwell improves treatment success.22
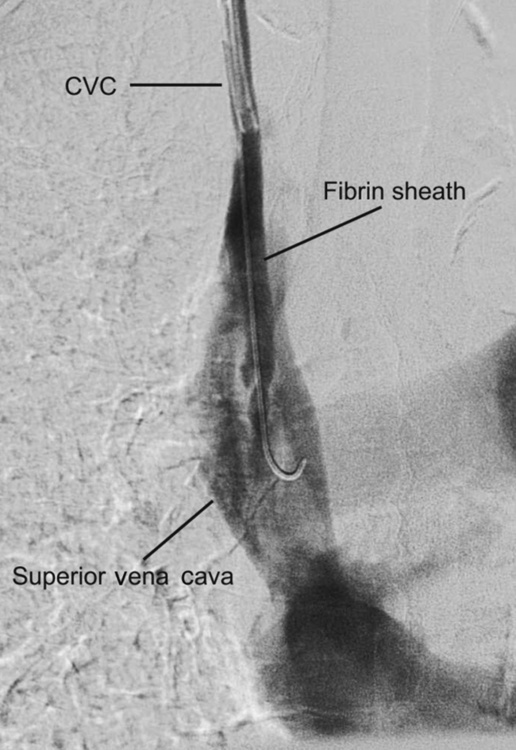
Fibrin sheaths
Fibrin sheaths cause up to 43% of catheter dysfunction.23 Contrast injection through the dialysis line may show a filling defect near the catheter tip or retrograde flow along the external surface of the catheter (Fig. 16.1). They may be treated by infusing a fibrinolytic agent over 6 hours, mechanical stripping using a snare from the femoral vein, or catheter exchange over a guidewire.
Stripping has a high technical success rate but the fibrin sheath frequently recurs. In a randomised trial there was no significant difference in additional patency between percutaneous stripping or urokinase.24 In another randomised trial 4-month catheter patency was significantly better after catheter exchange than percutaneous stripping.25
Catheter-related infection
Catheter-related infection is a major cause of morbidity and mortality and is related to the duration of placement. Gram-positive bacteria are the usual cause26 and resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA) are increasing. Catheter-associated sepsis can result in infective endocarditis, osteomyelitis, septic arthritis, epidural abscesses and death. Infection spreads either through the lumen of the catheter or along the outside from the exit site. An associated biofilm reduces the effectiveness of antibiotics.
Infections in non-tunnelled catheters should be treated by catheter removal and systemic antibiotics. In tunnelled catheters 90% of exit-site infections respond to oral antibiotics but intravenous antibiotics and catheter removal may be necessary for more serious tunnel infections. Systemic infections associated with tunnelled catheters can be treated initially with antibiotics but catheter removal is usually required. A new catheter should be inserted at a different site when the systemic sepsis has settled. Catheter exchange over a guidewire is controversial but there is some evidence that infection-free survival is similar to that after removal and delayed replacement.27
The cornerstone of prevention is scrupulous asepsis with regular exit-site inspection and dressing changes. Chlorhexidine and alcohol 70% provides superior asepsis to povidone–iodine 10% as an exit-site cleaning solution.28 Mupirocin ointment is also effective,29 but may increase colonisation by fungi and multiresistant organisms. Antibiotic-coated catheters reduce line sepsis in intensive care patients30 and temporary dialysis catheters31 but there is no evidence of benefit for long-term dialysis as the antibiotic is washed off over time. Bismuth has been demonstrated to have antibiofilm and antibiotic properties. A recent randomised clinical trial of bismuth-coated non-tunnelled dialysis catheters in 77 patients showed a reduction in catheter colonisation compared with non-coated catheters.32 There is also recent evidence that bacterial growth can be reduced by altering catheter surface irregularities.33 Antibiotic catheter locks have also been used and are effective at reducing catheter infections but there is a danger of antibiotic resistance. Antibiotic heparin and citrate heparin locks are superior to heparin alone but there are no randomised trials comparing antibiotic and citrate locks.34
Permanent dialysis access
Access planning
Whereas a side-to side radiocephalic AVF was originally described,41 an end-to side configuration is now preferred as there is less risk of peripheral venous hypertension. Some advocate an end-to-end anastomosis for distal radiocephalic AVFs, provided there is a good ulnar pulse, to reduce the small incidence of steal.42 Autogenous AVFs require a period of maturation to allow arterialisation of the venous outflow whereas AVGs can be needled directly as soon as the wounds have healed.
Preoperative assessment
There is an increasing trend for preoperative imaging to reduce primary failure, non-maturation and unnecessary surgery.35–37,43 This remains untested by clinical trial.
Duplex ultrasound
Preoperative duplex ultrasound is particularly useful for obese patients with impalpable superficial veins or following previous access failure, but cannot assess central vein patency. In studies from the USA, where AVGs are more frequently used than in Europe, routine preoperative ultrasound significantly increased the prevalence, reduced early failure rate and increased primary patency of autogenous AVFs compared with historical controls.44–46 Prosthetic AV access reconstruction and access complications also decreased significantly. In another study duplex mapping changed the proposed procedure in a third of patients and almost doubled the proportion of AVFs constructed.47 However, in a British study, duplex scanning rarely added any useful information except in those patients with poor vessels on clinical examination, when the proposed procedure was altered in 50%. This suggests that patients with good pulses and clinically adequate veins may proceed to surgery safely without preoperative ultrasound mapping.48
A radial artery luminal diameter of less than 1.6 mm is associated with early fistula failure49 and a minimum diameter of 2 mm is now usually advised.36,37,43 Above this threshold there seems to be no correlation between arterial diameter or flow and fistula success.
Venous diameter is an important determinant of outcome. In prospective studies, mean cephalic vein diameters are significantly smaller in non-functioning AVFs. Minimum venous diameters (with a tourniquet) of 2–2.5 mm have been advised for AVFs and 3.5–4.0 mm for synthetic grafts.36,37,43
Venous distensibility is another important predictor of success. Veins were found to dilate 48% with a tourniquet in successful fistulas compared with only 12% in fistulas that subsequently failed.50
Primary access
The snuffbox AVF is the most distal access possible and gives the longest length of vein for needling. It is possible in about 50% of patients and, in the event of failure, a wrist AVF can still be performed in half of the cases51 (Fig. 16.2).
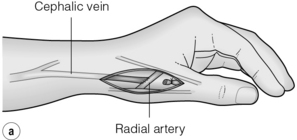
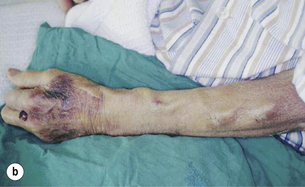
Figure 16.2 The snuffbox AVF. (a) Diagram showing position of anastomosis. (b) A mature snuffbox fistula showing the long length of available vein for needling.
The wrist radiocephalic AVF (Fig. 16.3) was the first to be described41 and remains the standard access in most units. It has a low complication rate and gives a good length of available vein. Patencies of 65% at 1 year are usual.52 If a wrist AVF fails, further forearm radiocephalic fistulas can often be created more proximally. In obese patients the vein may remain difficult to needle so that it may be advisable to excise the subcutaneous fat overlying the vein through one or more transverse incisions.
The brachiocephalic AVF is the next option, which can be performed in a variety of configurations (Fig. 16.4), and gives excellent flows at the expense of a greater incidence of steal (see below). To avoid this, some authors advocate anastomosing the cephalic vein to the radial artery 2 cm beyond its origin instead of the brachial artery itself.53
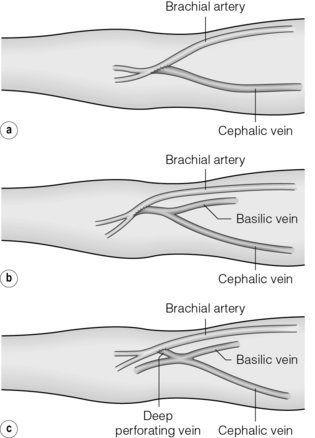
Figure 16.4 Configurations of the brachiocephalic AVF at the antecubital fossa. (a) Direct anastomosis between the cephalic vein and brachial artery. (b) Anastomosis including both median basilic and cephalic veins. (c) Gracz fistula between the deep perforating vein and the brachial artery.
The ulnobasilic AVF is often possible after a failed brachial fistula but is more difficult to needle and seems to have a poorer patency than other upper limb AVFs.54
When the options are limited by venous thrombosis or arterial disease an ulnocephalic or radiobasilic fistula may be possible in the forearm, but these require more extensive mobilisation and subcutaneous tunnelling of the vein across the forearm.55
Secondary and tertiary access
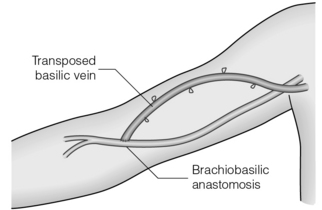
When the cephalic vein is thrombosed, an antecubital brachiobasilic AVF may be possible but this leaves only a short length of vein for needling so the basilic vein is usually mobilised and rerouted superficially over the biceps muscle either in a single procedure or in a second stage after the vein has arterialised (the basilic vein transposition; Fig. 16.5).56
When an autogenous AVF cannot be performed, a prosthetic graft (AVG) can be used. A variety of graft materials are available, but polytetrafluoroethylene (PTFE) is the most popular. The graft can be used for needling, after 2 weeks, and may be used sooner but this can be associated with perigraft haematoma formation. Grafts have a higher rate of infection compared to AVF. Thrombosis is much more common than in AVF and is usually due to intimal hyperplasia, at or just beyond the venous anastomosis. A wider graft,57 a vein cuff58 or an expansion of the venous end of the graft (Venoflo)59 may reduce this and has better patency. It is widely recognised that revision rates for grafts approach 80% annually compared to about 15% for AVF.
Biological grafts such as bovine mesenteric vein and bovine ureter are preferred in some units as they have greater resistance to infection, but they are prone to aneurysm formation.60 A forearm AVG either in a looped or straight configuration allows a basilic vein transposition to be performed if it fails, but has a poorer patency than the latter,61 so which should be performed first is a matter of dispute. A brachio-axillary AVG is the next option (Fig. 16.6).
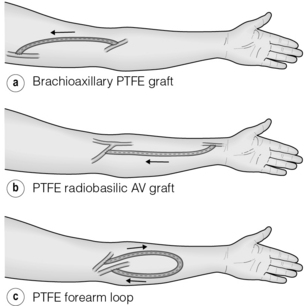
Figure 16.6 Popular configurations for upper limb AVGs: straight (a,b) and looped (c) brachio-axillary forearm grafts.
Lower limb access is less popular but may be the only option when the SVC or both subclavian veins are occluded. An AVF at the ankle between the greater saphenous vein (GSV) and the posterior tibial artery is rarely possible because of underlying arterial disease. The GSV can be anastomosed to the popliteal artery above the knee or used as a subcutaneous loop from the femoral artery in the groin, but these are more difficult to needle. Transpositon of the GSV in the thigh is less popular as it is more difficult to needle, but has fewer infective complications than synthetic thigh loop grafts.62 The superficial femoral vein can be used in the same configurations (or transposed to the forearm) and gives an excellent fistula at the expense of a high incidence of steal.
In desperate cases, a variety of possibilities exist: axillo-femoral, axillo-axillary, ilio-iliac or even aorto-IVC AVGs can be used. Where there are no available veins the RA can be used as an outflow. Alternatively an arterio-arterial graft, usually in the axillary position, is possible but risks distal embolisation to the arm.63 Occlusion of arterial interposition grafts may precipitate acute limb ischaemia.
Factors affecting access patency
The following factors are known to affect access patency:
• Vessel size. Small arteries and veins have higher initial failure rates, more frequent failure to mature and poorer long-term patency.49
• Fistula flow rate. The flow rate the day after surgery correlates inversely with the risk of thrombosis, although intraoperative flow rates are less reliable.49 The hyperaemic response of brachial artery blood flow is a strong predictor of access patency and maturation, presumably by detecting proximal arterial stenoses.64
• Mode of presentation. Patients presenting acutely with renal failure have poorer AVF patency, which may be linked to the need for temporary access via a central venous catheter.65
• Anastomotic method. Non-penetrating vascular clips, which give an interrupted anastomosis with excellent endothelial apposition and less bleeding, are quicker and have improved patencies compared with sutured anastomoses in randomised trials.66,67
• Access position. More proximal AVFs have improved patency67 but leave fewer options for access in the event of failure.
• Gender. Patency of AVFs is poorer in women than men.51,65,68,69
• Diabetes. There is conflicting evidence as to whether diabetes is an adverse factor, with some authors suggesting that AVF patency is poorer67 whereas others have found no effect.51,70,71
• Age. In a meta-analysis, access patency was found to be worse in the elderly.72 Wrist fistulas may perform as well as more proximal AVF.73
• Obesity. Veins are more difficult to cannulate in obese patients, which may account for poorer patency reported by some authors.74
• Smoking. Smoking reduces AVF patency.75
• Drugs. Antiplatelet agents such as aspirin and dipyridamole prolong fistula survival and are used routinely.76–78 A combination of aspirin and clopidogrel increased haemorrhagic complications without influencing patency in prosthetic AVGs in one study,79 but clopidogrel alone significantly prolonged graft survival in another.80 Warfarin reduces AVF thrombosis in patients with hypercoagulable states,81 but routine use is best avoided because of the risk of haemorrhage. Surprisingly, warfarin was associated with poorer patency in the Dialysis Outcomes and Practice Patterns Study (DOPPS), but this may reflect its use in patients with a history of fistula thrombosis or known thrombotic disorders.78 Calcium-channel blockers are associated with improved primary patency.78 Angiotensin-converting enzyme inhibitors did not affect primary patency in one study82 but were associated with improved secondary patency in DOPPS.77 Fish oil reduced AVF thrombosis in one randomised trial.8,83 Erythropoietin does not reduce and may increase patency, at least in AVGs.84,85
• Thrombotic tendencies and vasculitis. Increased fibrinogen and vasculitis predispose to access thrombosis.86
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree