Cellular Electrophysiology
The action potential (AP) is the fundamental electrophysiologic event in cardiac cells. The coordinated flux of ions into and out of the cardiac cell forms the basis for cardiac depolarization and repolarization. These ions flow through a series of ion channels, which are the object of intensive and ongoing investigation. It is increasingly appreciated that alterations in the function of these channels through inherited abnormalities, metabolic alterations, or drug modulation can result both in arrhythmia suppression and life-threatening proarrhythmia.
The AP differs in fundamental ways between tissues responsible for slow impulse conduction (nodal tissue) and those responsible for rapid impulse propagation (His-Purkinje system [HPS], ventricular myocardium). Furthermore, important differences exist between the AP of ventricular tissues which appear to be dependent on the layer of cells that are examined (endo-, mid-, and epicardial tissue layers).
Action Potential
Action Potential of Sinus Node and Atrioventricular Node
The AP of nodal tissues differs from the AP of other myocytes by its absence of a resting membrane potential. The sinus and atrioventricular (AV) nodes
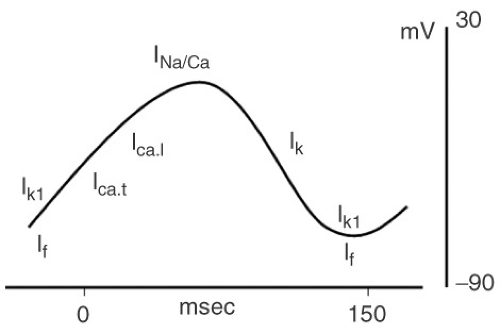
rely largely on calcium-dependent APs which have a slow upstroke and an absence of a resting potential (see Fig. 2-1). These slow response tissues (sinus node and AV node) do not depend on voltage-sensitive sodium channels to initiate cardiac depolarization. During diastole (repolarization) the membrane potential drops to −50 to −60 and then slowly and spontaneously depolarizes again. This spontaneous “pacemaker” current is conducted through a nonselective inward current called If (allows Na and Ca in and K out). Ik1 is also operative during this hyperpolarized potential and turns off as the cell is more depolarized. Depolarization is largely driven through slow voltage-gated inward calcium channels. There is a paucity of fast-activating sodium channels in nodal cells. Repolarization is achieved as the dominant current shifts to the outward (delayed rectifier) potassium current mediated by Ik.
rely largely on calcium-dependent APs which have a slow upstroke and an absence of a resting potential (see Fig. 2-1). These slow response tissues (sinus node and AV node) do not depend on voltage-sensitive sodium channels to initiate cardiac depolarization. During diastole (repolarization) the membrane potential drops to −50 to −60 and then slowly and spontaneously depolarizes again. This spontaneous “pacemaker” current is conducted through a nonselective inward current called If (allows Na and Ca in and K out). Ik1 is also operative during this hyperpolarized potential and turns off as the cell is more depolarized. Depolarization is largely driven through slow voltage-gated inward calcium channels. There is a paucity of fast-activating sodium channels in nodal cells. Repolarization is achieved as the dominant current shifts to the outward (delayed rectifier) potassium current mediated by Ik.
Atrial, His-Purkinje System, and Ventricular Myocytes
In contrast to nodal tissue, conduction in atrial and ventricular muscle as well as the His-Purkinje network occurs rapidly due to sodium-dependent APs (see Fig. 2-2). These fast-response tissues (atria, bundle of His, fascicles/bundle branches, terminal Purkinje fibers, and ventricular tissue) depend on these sodium channels under normal circumstances.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree