Wolff-Parkinson-White Syndrome and Variants
Ventricular preexcitation occurs in 0.1 to 3.1 out of 1,000 people, and is defined as activation of the ventricular myocardium by an atrial impulse earlier than would be expected with normal atrioventricular (AV) conduction. A delta wave is often seen on the surface electrocardiogram (ECG), which represents activation of the ventricle by an “accessory” pathway (AP) before activation by the conducting system (see Fig. 9-1). Wolff-Parkinson-White (WPW) syndrome is defined as an AP-mediated tachycardia occurring in patients with ventricular preexcitation on a 12-lead ECG.
APs occur when there is an incomplete segmentation of the embryologic cardiac tube and formation of the fibrotic AV ring during fetal cardiac development. The most common type of pathway is AV, formed by myocardial tissue connecting the atrium and ventricle, and most pathways are epicardial. AV pathways may be “manifest,” which means that they conduct antegradely from the atrium to the ventricle and result in preexcitation which can be seen on the surface ECG, or “inapparent,” which means that preexcitation is not seen on the surface ECG, or concealed because normal AV conduction activates the ventricle faster than the AP or because the AP does not conduct in an antegrade manner. These latter APs conduct only “retrograde” from the ventricle to the atrium, and are clinically relevant only when they participate in a tachycardia. In fact a minority of APs only conduct in the antegrade manner (preexcitation) whereas the majority conduct in a retrograde direction. Pathways exhibiting antegrade conduction do so in an “all or none” manner
99% of the time. Approximately 1% of antegradely conducting AV pathways exhibit decremental conduction, the vast majority of which are right sided.
99% of the time. Approximately 1% of antegradely conducting AV pathways exhibit decremental conduction, the vast majority of which are right sided.
 FIGURE 9-1. Diagram of antegrade conduction over both the normal atrioventricular (AV) conducting system and a left-sided accessory pathway. The amount of conduction over the accessory pathway corresponds to the degree of ventricular preexcitation or delta wave. (See color insert.) |
APs can be located anywhere around the A-V ring except at the portion of the aortomitral continuity where there is no ventricular myocardium below the atrium. They are often slanted, with the ventricular insertion point located closer to the septum and the atrial insertion more lateral in inferior APs and the ventricular insertion site lateral and atrial insertion site septal in anterior and posterior APs. Less common variants of typical AV APs are atriofascicular, nodofascicular, nodoventricular, and fasciculoventricular pathways, representing AP conduction between combinations of the atrium, AV node, conducting system, and ventricle. These variants are quite rare, but all except fasciculoventricular pathways may participate in tachycardias.
Clinical Evaluation
The first step in evaluating a patient who presents with preexcitation on an ECG is to take a thorough clinical history. The presence of symptoms associated with preexcitation often determines the course of the clinical evaluation. Symptoms may include sustained palpitations or syncope. A history of syncope must be taken carefully to differentiate neurocardiogenic or vasovagal syncope from
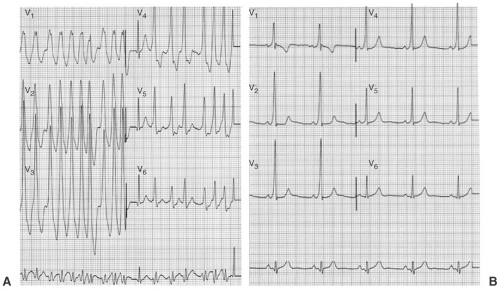
syncope related to an AP-mediated tachycardia (see Chapter 12). Syncope due to an AP will often be preceded by palpitations and may even require urgent cardioversion or defibrillation if rapidly conducted atrial fibrillation (AF) is present (see Fig. 9-2). Many patients will never have symptoms related to an AP, and the management of these patients is controversial (see discussion in the subsequent text). A family history of preexcitation or sudden cardiac death is important, as a familial association has been described. In addition, the presence of congenital heart disease should be ascertained. Ebstein anomaly is associated with right-sided APs, and when present the APs are often multiple and slowly conducting. Ebstein anomaly may be seen in “corrected” or L-type transposition of the great arteries in which the tricuspid valve (TV) is the left AV valve.
syncope related to an AP-mediated tachycardia (see Chapter 12). Syncope due to an AP will often be preceded by palpitations and may even require urgent cardioversion or defibrillation if rapidly conducted atrial fibrillation (AF) is present (see Fig. 9-2). Many patients will never have symptoms related to an AP, and the management of these patients is controversial (see discussion in the subsequent text). A family history of preexcitation or sudden cardiac death is important, as a familial association has been described. In addition, the presence of congenital heart disease should be ascertained. Ebstein anomaly is associated with right-sided APs, and when present the APs are often multiple and slowly conducting. Ebstein anomaly may be seen in “corrected” or L-type transposition of the great arteries in which the tricuspid valve (TV) is the left AV valve.
Asymptomatic Patients
The evaluation of patients presenting without identifiable symptoms or history of syncope and preexcitation on an ECG is controversial. The two risks to such patients are the development of an AP-mediated supraventricular tachycardia (SVT) and the occurrence of AF with rapid conduction over the AP leading to ventricular fibrillation and/or cardiovascular collapse. The incidence of the latter is extremely low (<0.02% per year), and while the magnitude of
this outcome warrants further risk stratification, this low risk should be stressed to the patient. The first step in risk stratification is noninvasive determination of the ability of the AP to conduct impulses rapidly from the atrium to the ventricle. If an AP is unable to conduct rapidly from the atrium to the ventricle, the risk of extremely rapid ventricular rates and ventricular fibrillation resulting from preexcited AF is low.
this outcome warrants further risk stratification, this low risk should be stressed to the patient. The first step in risk stratification is noninvasive determination of the ability of the AP to conduct impulses rapidly from the atrium to the ventricle. If an AP is unable to conduct rapidly from the atrium to the ventricle, the risk of extremely rapid ventricular rates and ventricular fibrillation resulting from preexcited AF is low.
It should be noted that APs have properties similar to myocardium (see subsequent text), and that in a setting of high adrenergic tone their ability to conduct rapidly increases. Therefore, the first step in noninvasive testing is often exercise treadmill testing because it induces a rapid heart rate in a setting of high adrenergic tone. If preexcitation is noted to disappear suddenly during exercise testing, the AP refractory period is likely long, and therefore it should be unable to conduct rapidly to the ventricle during AF. Care must be taken in reviewing the ECGs during stress testing; however, as the heightened adrenergic tone also increases AV conduction down the normal conduction system, and a decrease but not complete absence of preexcitation may be observed. The abrupt loss of the delta wave must be recognized to confirm that the refractory period of the AP is reached during routine exercise and is therefore unlikely to ever conduct AF at a potentially lethal rate.
Another noninvasive test that may be used to risk-stratify patients with asymptomatic preexcitation is a 24-hour Holter monitor. If preexcitation is noted to be intermittent on ambulatory monitoring, the AP refractory period is probably long and it is unlikely to be able to sustain rapid conduction during AF. Intravenous (IV) administration of procainamide (10 mg per kg over 5 minutes) has been used in the past to risk-stratify patients—disappearance of preexcitation with drug administration is associated with longer AP refractory periods. However, this test is rarely used in current clinical practice. The downside of these two tests is that neither evaluates the function of the AP in the setting of high catecholamines, and therefore may underestimate the capacity of an AP to conduct rapidly.
Patients who do not exhibit low-risk characteristics on noninvasive evaluation as described earlier may be offered invasive electrophysiologic testing. A frank discussion about the low risk of sudden death in patients with asymptomatic preexcitation and the comparably low risks of electrophysiology study is warranted at this point in the clinical evaluation. Factors that often determine whether invasive evaluation is pursued include high-risk occupations such as commercial drivers and pilots and, more commonly, patient preference. Some authors argue that patients who are asymptomatic and in the age-group of 35 to 40 years represent a low-risk group and do not warrant electrophysiologic (EP) testing, but because AF may develop later in life and is the presenting arrhythmia in up to 20% of patients presenting with WPW syndrome, this recommendation may not be justified. However, in the authors’ experience, they have not seen sudden death in this older age-group with preexcited AF, suggesting that the AP does not conduct at a rate conducive to the development of ventricular arrhythmia in this population. It should also be
noted that these same authors discount the utility of noninvasive testing and recommend EP testing in all patients with asymptomatic preexcitation who are younger than 35 years. The goals of EP testing are to evaluate the refractory period of the AP and to assess for inducible AP-mediated tachyarrhythmias. Specific methods are discussed in subsequent text.
noted that these same authors discount the utility of noninvasive testing and recommend EP testing in all patients with asymptomatic preexcitation who are younger than 35 years. The goals of EP testing are to evaluate the refractory period of the AP and to assess for inducible AP-mediated tachyarrhythmias. Specific methods are discussed in subsequent text.
Symptomatic Patients
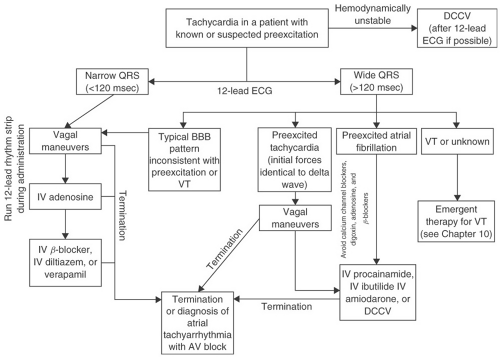
Patients presenting with symptomatic palpitations or syncope suggestive of a cardiac origin and preexcitation on an ECG should be offered an electrophysiology study for further characterization and often ablation of the AP (see subsequent text). This strategy is cost-effective and may allow a patient to avoid long-term medical therapy. For patients who do not wish to undergo invasive testing, drug therapy can be employed as a secondary approach. For patients with overt preexcitation, calcium channel blockers and digoxin should be avoided. Digoxin may shorten the refractory period of the AP, thereby allowing more rapid ventricular activation during AF and increasing the risk of ventricular fibrillation. Calcium channel blockers do not affect the refractory period of the AP in the baseline state, but have been shown to allow more rapid ventricular activation during AF when given intravenously, probably due to an increase in sympathetic tone secondary to hypotension induced by the medication or decreased retrograde concealment in the AP resulting from AV nodal slowing. The use of β-blocker is controversial, as these agents either do not affect or may even prolong AP refractoriness and slow the ventricular response in most patients with preexcited AF, but isolated reports of increased ventricular rates after their administration suggest that caution should be exercised in their use. Class IA and IC agents or amiodarone are the most effective at blocking conduction in the AP and preventing recurrences of documented tachycardia. Given the potential toxicities and proarrhythmic effects of these medications, symptomatic patients should be encouraged to undergo definitive treatment with ablation of the AP. Patients with tachycardia utilizing a concealed AP may be treated with β-blockers, calcium channel blockers, or digitalis. These medications slow conduction in the AV node and may suppress AV reentry. Figure 9-3 presents an algorithm for managing patients with known or suspected preexcitation who present with a tachycardia.
Electrocardiographic Interpretation
Evaluation of the 12-lead ECG of a patient with suspected preexcitation can provide significant information about the AP location. Algorithms have been proposed for the localization of APs, but none are >90% accurate and all have limitations. When interpreting a preexcited ECG, the duration of the PR interval and the vector of the delta wave are examined. In general, preexcitation caused by right-sided APs result in a shorter PR interval due to proximity to the
sinus node, with the terminal portion of the P wave often interrupted by the onset of the delta wave (see Fig. 9-4). APs excite the ventricular myocardium from the site of insertion at the base of the ventricle and activation spreads from this point. Therefore, the vector of the delta wave is determined by the site of ventricular insertion. As an example, a posterior (previously described as left lateral, see subsequent text) AP activates the posterior and lateral portion of the ventricle first and activation spreads anteriorly and to the right, resulting in a rightward axis of the delta wave and positive delta wave in the precordial leads (see Fig. 9-5).
sinus node, with the terminal portion of the P wave often interrupted by the onset of the delta wave (see Fig. 9-4). APs excite the ventricular myocardium from the site of insertion at the base of the ventricle and activation spreads from this point. Therefore, the vector of the delta wave is determined by the site of ventricular insertion. As an example, a posterior (previously described as left lateral, see subsequent text) AP activates the posterior and lateral portion of the ventricle first and activation spreads anteriorly and to the right, resulting in a rightward axis of the delta wave and positive delta wave in the precordial leads (see Fig. 9-5).
The traditional nomenclature describing APs was developed in the pathologic and surgical literature, and did not accurately locate the pathways as the heart sits in the chest cavity. Revised nomenclature has been developed which more accurately reflects the anatomic location of APs around the AV ring. Figure 9-6 depicts the location of APs using the revised nomenclature along with traditional designations. A synthesis of algorithms for localization of APs is presented in this figure, which can only be used as a general guide for localization. Factors which may affect ECG interpretation are multiple bypass tracts, rapid AV nodal conduction, intra-atrial conduction defects, hypertrophy, congenital heart disease, and prior myocardial infarction. More
accurate localization and characterization of APs requires an electrophysiologic study.
accurate localization and characterization of APs requires an electrophysiologic study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree