Permanent Pacemakers
Clinical Indications
The clinical indications for permanent pacemaker (PPM) implantation are covered in detail in Chapter 11. A brief recapitulation is provided in the subsequent text.
Sinus Node Dysfunction
Sinus node dysfunction is the most common indication for pacemaker implantation, accounting for 40% to 60% of all implants. Symptomatic sinus node dysfunction (i.e., documented sinus bradycardia or sinus pauses with symptoms) as well as chronic chronotropic incompetence are a class I indication for PPM implantation. Less commonly, asymptomatic patients may require pacing for sinus node dysfunction. Pharmacologic therapy to treat sinus node dysfunction is limited and because many patients require medications that can suppress sinus node function (such as β-blockers, calcium channel blockers, or antiarrhythmia medications), pacemaker implantation may be required to treat sinus node dysfunction exacerbated by external (medication) causes. Often patients with sinus node dysfunction have accompanying supraventricular arrhythmias and the incidence of developing atrial fibrillation is 5% per year.
Pacing for Atrioventricular Block
Atrioventricular (AV) block is the second most common indication for pacemaker implantation. As detailed in Chapter 11, common causes include heightened vagal tone, medications, senile degeneration, infection, and infarction.
Pacing for Other Indications
Pacemaker implantation is occasionally utilized to treat other disorders. Neurocardiogenic syncope associated with bradycardia (particularly sinus arrest) documented spontaneously or during tilt table testing that is recurrent and unresponsive to medical treatment may benefit from PPM implantation although several large randomized trials have revealed conflicting results. Patients receiving a pacemaker for this indication may be treated with rapid pacing (e.g., at ∼100 bpm) for a short period of time (i.e., 2 minutes) upon onset of vagal symptoms and may have fewer episodes of syncope (although, patients may still become highly symptomatic from a vasodepressor response).
Some patients undergo pacemaker implantation for ventricular resynchronization to treat heart failure. Candidates for this therapy are those patients with ischemic or nonischemic cardiomyopathy, reduced left ventricular ejection fraction (≤35%), heart failure symptoms despite optimal medical therapy, and left bundle branch block. These patients may have asynchronous activation of the septum and delayed activation of the left lateral wall resulting in a reduced stroke volume and often increased mitral regurgitation. Currently, the evidence for echocardiographic identifiers of interventricular dyssynchrony (dyssynchronous contraction of the right and left ventricles) is equivocal.
Randomized trials have demonstrated that some patients have improved clinical outcomes (quality of life, exercise capacity, maximum oxygen consumption, reduced heart failure hospitalizations, and improved survival) with biventricular pacing (simultaneous right and left ventricular pacing). Left ventricular pacing is performed by stimulating through an additional electrode that is placed transvenously through the coronary sinus to the epicardial LV (although occasionally the LV lead is placed on the epicardial surface of the heart through a surgical or thoracoscopic approach). Typically, the best place for left ventricular pacing is the posterolateral or lateral cardiac veins (see Chapter 1).
Dual chamber pacing is occasionally used for hypertrophic obstructive cardiomyopathy. Right ventricular pacing changes the septal activation sequence and therefore may induce dyssynchrony and decrease the outflow tract gradient. Results from clinical trials have found that the effects are at best modest—and in some cases may even be detrimental. Pacing may be beneficial to allow uptitration of β-blockers or calcium channel blockers for their lusitropic effect or to allow AV junctional ablation in the setting of poorly controlled atrial fibrillation.
Pacing may be beneficial in some patients with the long QT syndrome who present with bradycardia or pause-induced torsade de pointes.
Pacemaker Features
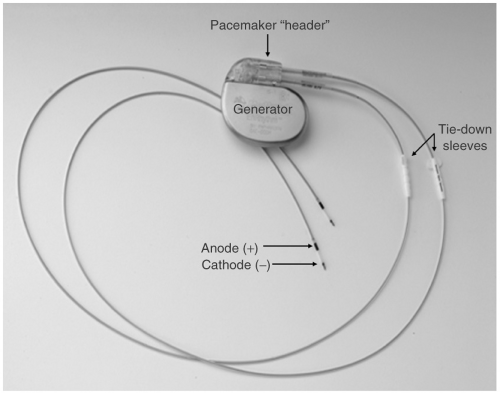
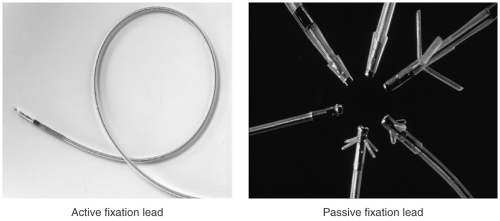
A pacemaker system consists of the pulse generator and lead(s) (see Fig. 15-1). The pulse generator consists of the device circuitry and battery whereas the pacemaker lead consists of the electrode, insulation, connector pin, and a fixation mechanism. The lead conducts the cardiac signals to the pulse generator in either a bipolar (two electrodes at the tip of the lead in the cardiac chamber) or unipolar (a single electrode at the tip of the lead with the pacemaker generator “can” functioning as the second electrode) configuration. Most modern implanted electrodes are bipolar because they are less prone to sensing noncardiac electrical signals. Several fixation mechanisms are available including passive fixation with tines, wings, or fins or active fixation using exposed or retractable screws (see Fig. 15-2). Passive fixation leads are anchored to the heart by entrapment to trabecular muscle whereas active fixation mechanisms allow the placement of the leads in any position of the heart.
Single-chamber pacemakers are most often utilized for patients with chronic atrial fibrillation (in whom sensing or pacing in the atrium is unnecessary). Although, single-chamber atrial pacing is still performed on some
occasions, most patients with sinus node dysfunction receive dual-chamber pacemakers due to the common association of sinus node dysfunction with AV node dysfunction. In fact, patients with sinus node dysfunction but no evidence of AV nodal disease at the time of pacemaker implantation develop significant AV node disease at a rate of 0.6% to 2.7% per year. It is the authors’ practice to implant dual rather than single-chamber pacemakers in most patients except in those with chronic atrial fibrillation.
occasions, most patients with sinus node dysfunction receive dual-chamber pacemakers due to the common association of sinus node dysfunction with AV node dysfunction. In fact, patients with sinus node dysfunction but no evidence of AV nodal disease at the time of pacemaker implantation develop significant AV node disease at a rate of 0.6% to 2.7% per year. It is the authors’ practice to implant dual rather than single-chamber pacemakers in most patients except in those with chronic atrial fibrillation.
Pacemaker batteries are expected to last for 6 to 12 years. The duration of battery life is related to the amount of pacing that occurs (single vs. dual chamber and continuous vs. intermittent). It is also related to the amount of energy needed to capture the respective chamber. All pacemakers have indicators signaling when the elective replacement time is reached. Common indicators include (a) a decrease in pacing rate upon magnet application, (b) change to a simpler pacing mode (from DDDR to VVIR), (c) reduced battery voltage, (d) increase in battery impedance, and (e) loss of programmability. If the pacemaker battery reaches end of life the pacemaker changes to the simplest pacing mode and may fail to communicate.
Pacing Modes
The North American Society of Pacing and Electrophysiology (NASPE) and the British Pacing and Electrophysiology Group have established a five-letter code which is periodically updated (see Table 15-1). The code is generic and applies to all pacemaker makes and models.
VVI
In VVI mode, pacing and sensing occur only in the ventricle. Any paced or sensed event starts an internal timer—the expiration of the lower rate interval
(LRI) without an intervening sensed event (i.e., ventricular beat) will cause the pacemaker to deliver a pacing impulse and reset the lower rate timer. A sensed event will also restart the lower rate timer and therefore will inhibit the pacemaker from delivering a paced beat. VVI pacing is most often used in patients with chronic atrial fibrillation.
(LRI) without an intervening sensed event (i.e., ventricular beat) will cause the pacemaker to deliver a pacing impulse and reset the lower rate timer. A sensed event will also restart the lower rate timer and therefore will inhibit the pacemaker from delivering a paced beat. VVI pacing is most often used in patients with chronic atrial fibrillation.
TABLE 15-1 The Revised North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group (NASPE/BPEG) Generic Code for Antibradycardia, Adaptive-Rate, and Multisite Pacing
| Position | I | II | III | IV | V |
| Category | Chamber(s) Paced | Chamber(s) Sensed | Response to Sensing | Rate Modulation | Multisite Pacing |
| O = None | O = None | O = None | O = None | O = None | |
| A = Atrium | A = Atrium | T = Triggered | R = Rate modulation | A = Atrium | |
| V = Ventricle | V = Ventricle | I = Inhibited | V = Ventricle | ||
| D = Dual (A + V) | D = Dual (A + V) | D = Dual (T + I) | D = Dual (A + V) |
AAI
AAI mode is identical to VVI mode, except that pacing and sensing occur in the atrium, not in the ventricle.
DDD
In DDD mode (see Fig. 15-3), pacing and sensing occur in both the atrium and ventricle. In addition, a sensed or paced beat in the atrium “triggers” pacing in the ventricle if intrinsic conduction does not occur spontaneously after a programmed delay.
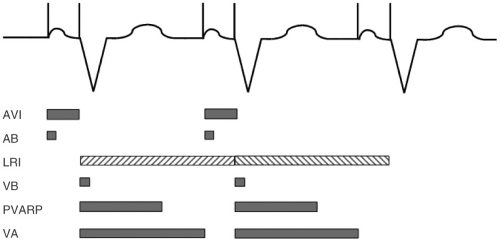
Atrioventricular Interval
Most pacemaker timing intervals are based on the ventricle. A sensed or paced event in the ventricle will start the LRI timer. At the same time, a second timer for the VA interval is started. If no atrial event is sensed at the end of the VA interval a pacing impulse will be delivered in the atrium. An atrioventricular interval (AVI) is started at that time and a ventricular pacing spike will
be delivered if no ventricular event is sensed. The sum of the VA interval and AVI is the LRI.
be delivered if no ventricular event is sensed. The sum of the VA interval and AVI is the LRI.
Postventricular Atrial Refractory Period
Two important programmable timers are started upon sensing or pacing events in the ventricle. The ventricular refractory period avoids T-wave oversensing. At the same time, the postventricular atrial refractory period (PVARP) timer is started. During that time no atrial sensing occurs in order to prevent sensing of retrogradely conducted P waves in the atrium, which may result in pacemaker-mediated tachycardia (PMTs) (see subsequent text). The upper tracking rate is limited by the sum of the PVARP and the AVI.
Cross Talk and Safety Pacing
Many pacemakers have the capability to shorten the AVI with increasing heart rates. Usually the sensed AVI is shorter than the paced AVI. After a paced atrial stimulus, the AVI is divided into two parts: A programmable blanking period starting with the atrial stimulus and followed by sensing in the ventricle during the remainder of the AVI. During the blanking period no sensing in the ventricles occurs in order to avoid sensing of the atrial stimulus for a ventricular event which would cause inhibition of ventricular pacing (cross talk inhibition). A sensed event in the ventricle during the second part of the AVI (possibly caused by a ventricular premature complex [VPC] or the atrial stimulus) will result in a ventricular safety pacing stimulus usually with an AVI of 100 msec (see Fig. 15-4). This ensures that no cross talk inhibition
occurs which could otherwise result in asystole in pacemaker-dependent patients.
occurs which could otherwise result in asystole in pacemaker-dependent patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree