Atrial Flutter
Clinical Characteristics
Atrial flutter is a regular, macro reentrant arrhythmia with an atrial rate of 250 to 320 beats per minute (bpm). The pathophysiology of atrial flutter is closely related to that of atrial fibrillation. The incidence of atrial flutter has been estimated at 0.88% of the general population, increasing to 5.87% in patients older than 80 years of age (see Table 7-1).
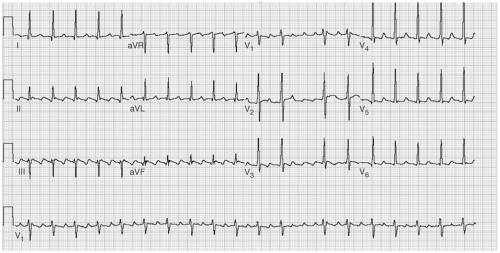
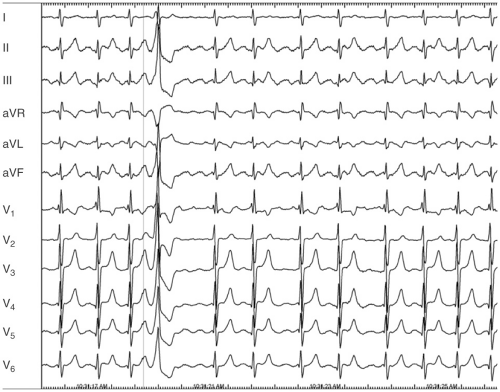
One of the difficulties in understanding atrial flutter is related to terminology. The term atrial flutter is used to describe several different macro reentrant arrhythmias, which differ in location and pathophysiology. These distinctions, although initially confusing, are particularly important with regard to ablative therapy for atrial flutter. The most frequent way of classifying atrial flutter is isthmus dependent (or typical) and non–isthmus dependent. Isthmus-dependent atrial flutter describes a right atrial circuit, bounded anteriorly by the tricuspid annulus and posteriorly by the caval veins, crista terminalis, and the Eustachian ridge (see Fig. 7-1). This circuit is most confined in the low atrium, as it passes around the inferior vena cava and the Eustachian ridge. These structures, with reference to the tricuspid valve, are the “isthmus” through which atrial flutter must pass and form the target for ablation therapy. Isthmus-dependent flutter has “sawtooth” flutter waves in the inferior leads (see Fig. 7-2). Viewing the atrium with the tricuspid valve turned up like a clockface, atrial flutter can traverse the same circuit in a clockwise (in which
case the inferior leads are positive) or a counterclockwise (in which the inferior leads are negative) direction (see Table 7-2).
case the inferior leads are positive) or a counterclockwise (in which the inferior leads are negative) direction (see Table 7-2).
TABLE 7-1 Electrocardiographic (ECG) Characteristics of Atrial Flutter
| SVT, supraventricular tachycardia. |
| Be suspicious of any regular SVT with a ventricular rate of ≈150 bpm |
|
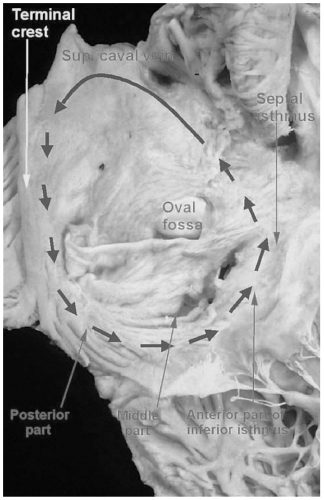 FIGURE 7-1. Anatomy of the right atrial flutter circuit. (See color insert.) |
Non–isthmus-dependent flutter refers to regular, macro reentrant atrial arrhythmias that occur outside of the isthmus, typically in regions of atrial scarring (see Fig. 7-3). This scarring may occur spontaneously, for reasons that are not well understood, or as the result of surgical incisions or extensive atrial
ablation. In addition to natural barriers (such as the veins that enter the atria, or the valve annuli), scarring provides the boundaries for establishing atrial flutter circuits, as well as the requisite slow conduction. There is some overlap, both in atrial rate and electrocardiographic appearance between non–isthmus-dependent atrial flutter and pulmonary vein–related atrial tachycardia, which is probably due to reentry on a much smaller scale. Because of the wide
variability of this process and its relative rarity (at least before atrial fibrillation ablation procedures), this chapter will focus on isthmus-dependent atrial flutter.
ablation. In addition to natural barriers (such as the veins that enter the atria, or the valve annuli), scarring provides the boundaries for establishing atrial flutter circuits, as well as the requisite slow conduction. There is some overlap, both in atrial rate and electrocardiographic appearance between non–isthmus-dependent atrial flutter and pulmonary vein–related atrial tachycardia, which is probably due to reentry on a much smaller scale. Because of the wide
variability of this process and its relative rarity (at least before atrial fibrillation ablation procedures), this chapter will focus on isthmus-dependent atrial flutter.
TABLE 7-2 Diagnosis of Isthmus-Dependent Atrial Flutter during Electrophysiologic (EP) Studies
| CS, coronary sinus. |
|
Atrial flutter most often occurs in patients with structural heart disease, as some degree of right atrial enlargement and/or conduction slowing is required to make atrial flutter possible. One way of expressing this point is consideration of the wavelength, which is defined as the distance that the arrhythmia wave front travels in the average refractory period of the atrial tissue, or the conduction velocity × refractory period. In order for atrial flutter to occur, the wavelength must be shorter than the path length of the anatomic circuit or else the wave front would run into its refractory wake and extinguish. Anything that makes the wavelength shorter (fibrosis, antiarrhythmic drugs that block sodium channels) or the atrium larger (valvular heart disease, arrhythmia-induced remodeling, hypertension, cardiomyopathy) will facilitate atrial flutter.
There is a wide range of symptoms caused by atrial flutter, mostly dependent on ventricular rate, ranging from none to syncope. Characteristically, the ventricular rate has an even integral relationship with the atrial rate, usually 2:1. This results in a typical ventricular rate of 150 bpm, as the atrial rate is normally 300 bpm. Patients typically perceive this as rapid palpitations or as exercise intolerance because the rate is inappropriately high and does not change with increasing workload. Exercise intolerance and fatigue can occasionally occur because the ventricular rate is inappropriately slow, as would be observed in 8:1 flutter (ventricular rate ≈40 bpm). In other circumstances, there are no symptoms, perhaps owing to the fact that the atrial rhythm and more importantly the ventricular response, is regular. Atrial flutter may be paroxysmal, persistent, or may degenerate to atrial fibrillation.
Under unusual circumstances, the ventricular response to atrial flutter can be 1:1, a situation that has a high likelihood of causing syncope. The typical situation that leads to this is the use of sodium channel blocking antiarrhythmic drugs for atrial flutter or atrial fibrillation without atrioventricular (AV) nodal blocking agents. The antiarrhythmic drug slows the atrial rate during atrial flutter, and in some cases (classically with quinidine) facilitates AV conduction.
Atrial flutter and atrial fibrillation are interconnected on many levels. The pathophysiology of both are similar, and the two conditions coexist in many patients. Both arrhythmias are progressive, which is thought to be due to arrhythmia-related “remodeling” on structural (increased atrial size), electrical (shortening of the atrial refractory period), and ultrastructural (promoting atrial fibrosis) levels. Furthermore, the remodeling caused by one arrhythmia leads to progression of the other. Approximately 15% of patients treated with class IC antiarrhythmic agents or amiodarone for atrial fibrillation will develop atrial flutter, often exclusively. This observation led to the development of a “hybrid” management strategy of antiarrhythmic drugs to treat atrial fibrillation, and ablation to treat atrial flutter. On the other hand, approximately 50% of patients with “pure” atrial flutter will develop atrial fibrillation over 5 years. This progression occurs despite adequate treatment for atrial flutter and seems to be higher if episodes of atrial flutter continue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree