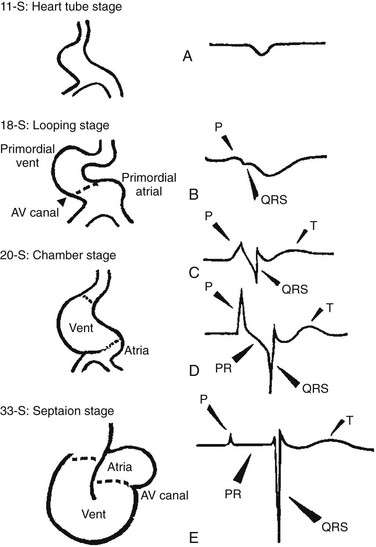
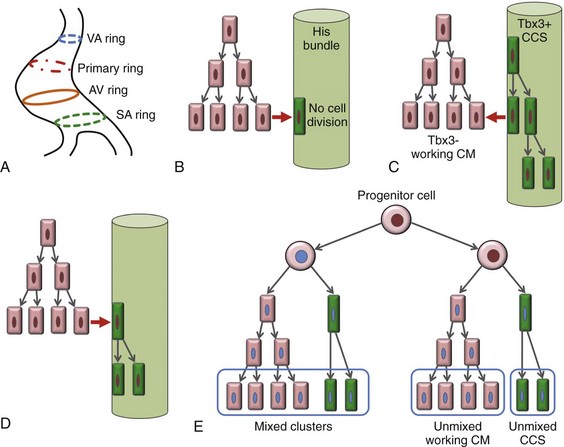
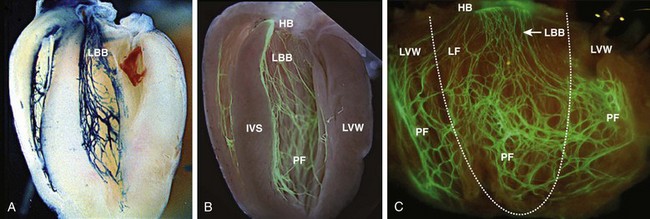
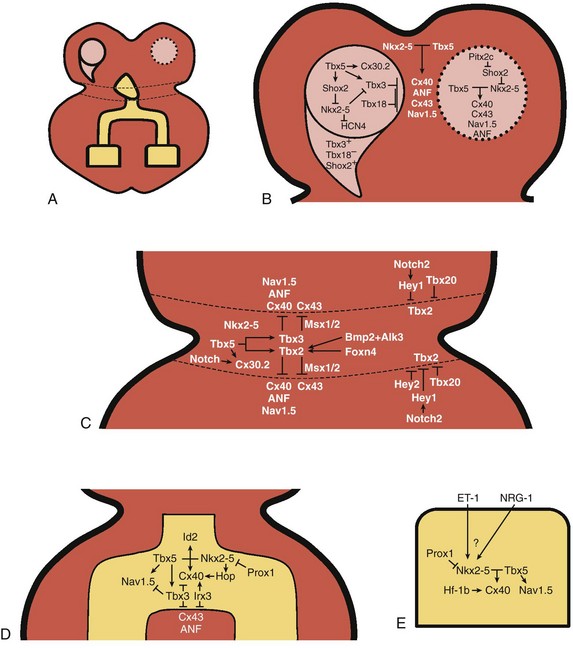
29 The framework of the cardiac conduction system is laid down early during heart development. Paff et al.1 noted that the chick electrocardiogram (ECG) transforms from a sinusoidal waveform to the mature configuration before identifiable components of the cardiac conduction system are formed (Figure 29-1).1 They also noted that atrioventricular (AV) block was achievable with digitalis at the 18-somite stage before a discernible PR interval was evident, suggesting that the AV nodal primordium develops within the early heart tube (see Figure 29-1, B).1 After the 18-somite stage, cardiac chamber formation ensues and the ECG begins to manifest evidence of fast conduction by the presence of high frequency P waves and QRS complexes (see Figure 29-1, C and D). The evolution of the chick ECG clearly demonstrates that the slow conducting nodal elements are present in the early looped heart and that the fast conducting elements are incorporated during chamber formation. The attainment of a mature ECG configuration before structural maturation of the CCS is achieved by interposing slowly conducting AV canal myocardium between the fast conducting atrial and ventricular chamber myocardium (Figure 29-1, E). The fast-conducting components are enriched in high-conductance gap junction proteins, connexin40 (Cx40), and the α-subunit of the cardiac sodium channel, Nav1.5 (encoded by Scn5a), whereas the AV canal expresses low-conductance gap junction proteins, Cx30.2 and Cx45.2 How these electrophysiologically distinct regions are specified and what defines the boundaries between slow and fast conduction have been the focus of intense research over the past 50 years. This chapter discusses the developmental origins of the cardiac conduction system and the transcriptional networks that govern its formation. Figure 29-1 Schematic of chick heart development with corresponding electrocardiograms at the somite stage. A, 11. B, 18. C and D, 20. E, 33.1 Viragh and Challice3–5 performed meticulous histologic analysis of the developing cardiac conduction system in mouse embryos between 8 and 12 days after coitus (E8-E12). Conduction cells were distinguished from working cardiomyocytes by the following characteristics: (1) periodic acid–Schiff (PAS) positive staining, (2) poorly organized contractile apparatus; (3) enriched glycogen content, and (4) reduced number of T-tubules. Using these features, the temporal-spatial distribution of conduction cells was then tracked during cardiac development.3–5 In the developing mouse embryo, the contractile sequence of the heart is established by E9, well before the appearance of the sinoatrial node primordium at E11.5 The origin of contraction was noted to be in the right sinus horn. Within the dorsolateral wall of the sinus horns, loose mesenchymal cells were noted to transform into the early sinus musculature, which extends along the sinus side of the sinoatrial venous valves. This aggregation of early sinus muscle tissue was the presumed site of SAN development and corresponded to the site that Wenink termed the “sinoatrial (SA) ring.”6 The SAN primordium was recognizable at E11 in the medioanterior wall of the right superior vena cava within the early sinus muscle. A left-sided SAN develops simultaneously in the medioanterior region of the left common cardinal vein, but ultimately resorbs and incorporates into the wall of the left atrium.5 In humans, the SAN primordium is a long structure located within the subepicardial cleft at the site of invagination of the sinus venosus and the right atrium, which forms the right venous valve.5 The sinoatrial (SA) and AV conduction system develop simultaneously. At E9 to E10, the AV canal (AVC; i.e., AV ring) is a well-defined constriction, and the inner cell layer of the AVC makes numerous interconnections with the trabecular compartment, which is the source of the His-Purkinje system (HPS).3,4 At E11, the primordium of the AVN was identified as a PAS+ cell cluster in the inner, dorsal AVC. These PAS+ AVC cells were contiguous with the crest of the developing interventricular septum (primary ring), positioning the AV nodal anlage in direct communication with the primordial His bundle and bundle branches. During this time, the outer cell layer of the AVC was undergoing apoptosis. In the trabecular region, glycogen-rich, PAS+ cells were seen immediately subjacent to the endocardium; these nascent Purkinje cells formed extensive connections with the developing bundle branches.3,4 Therefore, all components of the AV conduction system are in contact with each other throughout cardiogenesis, indicating that an initial framework for the mature conduction system is in place in the early heart. The work by Viragh and Challice3–5 clearly demonstrated that conduction system development is inextricably linked to cardiogenesis. Yet, significant questions remained regarding the cellular origins of the conduction system, the mechanism by which the pool of conduction cells expands, and the factors that dictate CCS specification and patterning. The neuronal qualities of the cardiac conduction system led many to believe that its cellular origins were from neural crest derivatives. However, lineage-tracing studies in the chick and mouse demonstrated that all conductive components of the CCS are myocardial in origin.7–9 Using a replication-defective retrovirus expressing LacZ, individual myocytes were labeled in developing chick hearts before neural crest immigration, and the expression of β-galactosidase (β-gal) was traced in daughter cells.7 Single myocyte clones gave rise to both conduction cells and working cardiomyocytes. Labeling of neural crest cells, however, failed to show any incorporation into the CCS.7,8 Similar findings were reported in the murine heart using retrospective clonal analysis, where individual myocyte progenitor clones gave rise to conduction and nonconduction cells.9 Similar to the chick, lineage-tracing studies in mice have failed to identify a direct contribution of neural crest cells to the specialized conduction system.10,11 The prevailing models of conduction system development are the ring model,6 the inductive recruitment or in-growth model,7 the early specification or outgrowth model,12 and the biphasic model (Figure 29-2). The ring hypothesis was based on early observations that the specialized conduction system formed within four constriction points in the D-looped heart (see Figure 29-2, A).6 Four rings were noted to form from the venous pole to the arterial pole: the SA ring, AV ring, primary (interventricular) ring, and ventriculoarterial (VA) ring. These constriction areas are created by differential proliferation rates that exist between ring myocardial cells and flanking chamber cardiomyocytes. Although these areas of constriction exist within the developing heart during chamber formation, the ring hypothesis has largely been discredited, as there is no evidence to support a preexisting template for ring formation within the tubular heart. It is now known that the linear heart tube is composed of clonally related myocardial cells from the first heart field.13 Figure 29-2 Models of cardiac conduction system development. A, Ring model. Prespecification of conduction system components within the linear heart tube. B, Inductive recruitment or ingrowth model. Undefined inductive signals recruit proliferating cardiomyocytes to a conduction lineage, then cease to proliferate (red arrow). C, Early specification or outgrowth model. Tbx3+ CCS cells expand from primitive myocardium that retains a conduction phenotype. Loss of Tbx3 (or Tbx2) expression results in a phenotypic change from a CCS cell to a working cardiomyocyte, the default pathway (red arrow). D, The biphasic model incorporates both the ingrowth and outgrowth models. E, Retrospective clonal analysis of LacZ-labeled, cardiomyocyte clones (blue nuclei). Mixed clusters of conduction and working cardiomyocytes are consistent with a common myocardial progenitor. Unmixed clusters of CCS-only cells demonstrate the potential for limited rounds of cell proliferation. (From Miquerol L, Beyer S, Kelly RG: Establishment of the mouse ventricular conduction system. Cardiovasc Res 91:232–242, 2011.) Cheng et al.7 put forth the recruitment model or “in-growth model” based on several observations noted during chick VCS development (see Figure 29-2, B).7 First, the proliferative capacity of developing VCS components was found to be significantly lower than working myocytes based on pulse labeling experiments using [3H]-thymidine. Once specified, conduction cells appeared to exit the cell cycle and become quiescent. Second, lineage tracing studies showed that individually labeled myocyte clones gave rise to conduction cells and working myocytes. Third, cell birth dating experiments demonstrated that new conductive cells were added to the developing His bundle in lamellar fashion, analogous to tree rings. These observations led the authors to conclude that the specialized conduction system expands through a process of inductive recruitment of neighboring myocytes.7 However, it was not speculated what constituted the early framework upon which new conduction cells were added or the nature of the molecular signal used for inductive recruitment. The early specification model, or outgrowth model, states that conduction cells expand from a progenitor pool that retains its specialized conduction phenotype (see Figure 29-2, C).12,14 The conduction gene programming is retained by the expression of transcriptional repressors that suppress a working myocardial phenotype, which is the default pathway. In support of this hypothesis, persistent expression of repressive transcription factors (Tbx2, Tbx3, Msx2, and Id2) has been identified within primordial conduction regions.12,14,15 Tbx3 is expressed as a continuous band linking the SAN and AVN, internodal tracts, and the proximal ventricular conduction system.12 Heterologous expression of Tbx2 or Tbx3 is able to suppress chamber-type myocardial genes (Nppa [ANF], Gja1 [Cx43], Gja5 [Cx40]), inhibit cardiac chamber formation, and in the case of Tbx3 elicit ectopic pacemaker formation.12,14–16 Consistent with these findings, Tbx3 and Cx43 exhibit complementary expression patterns in the developing heart.12 Most recently a biphasic model of conduction system development has been proposed (Figure 29-2D).9 In this model, once conduction cells are recruited from myocardial precursors, they retain the capacity to undergo limited rounds of cell division. Analysis of labeled myocyte clones revealed two classes of conductive clusters, mixed and unmixed (see Figure 29-2, E). The mixed clusters represented single myocyte clones that gave rise to both conductive and working myocytes (recruitment). The unmixed clones were composed of either working myocytes or conduction myocytes, but not both. Conduction-only, unmixed clones were identified throughout the central and peripheral VCS, indicating that once specified, all components of the conduction system are capable of approximately four to five rounds of cell division. Based on these findings, the authors concluded that mammalian VCS development appears to use both in-growth and outgrowth modes of expansion.9 However, these findings are not incompatible with the early specification/outgrowth model, because mixed clusters might represent conduction cells that have lost Tbx2/Tbx3 expression and have defaulted to the chamber pathway. Visualization of the developing CCS has been greatly enhanced by the development of conduction system reporter mice (Figure 29-3). Each reporter mouse delineates different components of the CCS at various developmental time points using LacZ or green fluorescent protein (GFP) expression. The CCS-LacZ and minK-LacZ mouse lines are representative examples of well-established markers of the specialized conduction system.17–19 The CCS-LacZ mouse was created serendipitously through a complex genomic rearrangement involving the MC4/engrailed-2-LacZ cassette (see Figure 29-3, A).18 CCS-LacZ reporter expression can first be detected at E8.5 in the SAN primordium within the venous pole. At subsequent stages, β-gal expression is detected in the developing and mature AVN and His-Purkinje system. All CCS reporter lines have some degree of cardiac expression outside of the conduction system. In the adult CCS-LacZ heart, significant β-gal expression is seen within the right atrium.18 Figure 29-3 Cardiac conduction system reporter mice. A, CCS-LacZ. B, Contactin2-eGFP. C, Connexin-40–eGFP.20 The minK-LacZ reporter mouse was created by replacing the minK gene with a nuclear-targeted LacZ cassette.17 Early in development, β-gal expression was noted in the SA ring, AV ring, interventricular ring, and the VA ring. Subsequently, β-gal expression was confined to the AVN and the proximal conduction system, as well as in the venous valves, AV ring, and VA valves.17,19 The Cx40-eGFP reporter mouse has become a widely used tool to characterize normal and abnormal patterning of the mature His-Purkinje system (see Figure 29-3, C).20 Developmentally, Cx40 expression is not restricted to the VCS, with significant expression in the trabecular myocardium. In addition, Cx40 is not expressed in the distal AVN or His bundle before E14.5. In the mature heart, Cx40 is enriched in atrial myocardium and in coronary endothelial cells.20 Contactin-2 (Cntn-2) was recently identified as a CCS-enriched factor using differential gene profiling of adult mouse Purkinje fibers versus working myocytes (see Figure 29-3, B).21 Cntn-2 is a cell adhesion molecule that has a role in neuronal patterning and ion channel clustering. Both Cntn2-LacZ knock-in mice and Cntn2-EGFP BAC transgenic reporter mice delineated the entire cardiac conduction system in postnatal hearts. Currently, a functional role for Cntn-2 in the CCS has not been identified.21 A rich hierarchy of gene networks dictates the specification and patterning of conduction components (Figure 29-4). Unifying all these networks is the balance struck between prochamber myocardial programming versus antichamber programming. As mentioned previously, the T-box transcription factors dictate much of this equilibrium, tilting the scales toward or away from a conduction lineage. The T-box factors can function as transcriptional activators or repressors and are known to be critical regulators of cardiac specification and differentiation.22 Seven TBX family members are expressed in the developing heart, four of which (TBX1, TBX5, TBX20, TBX3) have been linked to human congenital heart disease.23–25 The major cardiac transcriptional activators, Tbx5 and Tbx20, act through Nkx2-5 and Gata4 to drive prochamber myocardial gene expression, such as Nppa (ANF) and Gja5 (Cx40).26–28 The transcriptional repressors Tbx2, Tbx3, and Tbx18 compete with Tbx5 for Nkx2-5 binding to suppress chamber-specific gene expression, thus maintaining a conduction gene profile.12,14,15,29,30 Figure 29-4 Transcription factor regulatory networks. A, Schematic of the cardiac conduction system. B, Right sinoatrial node (head and tail domains), atrial myocardium, and left sinoatrial node (stippled circle). C, Atrioventricular canal/node region flanked by atrial and ventricular myocardium. D, His bundle and bundle branches and ventricular myocardium. E, Purkinje fiber. The delicate balance between Tbx activators and repressors and the expression of cofactors dictates the rich conduction system phenotypes seen in the mature CCS. Sitting on top of the CCS specification hierarchy is Tbx5, a critical determinant of many elements of the slow and fast conduction system.31–33 Mutations in Tbx5 result in Holt-Oram syndrome, an autosomal dominant condition characterized by preaxial radial ray limb deformities and cardiac septation defects.22 The septal defects are typically ostium secundum atrial septal defects, muscular ventricular septal defects, and atrioventricular canal defects. Patients with Holt-Oram syndrome manifest variable degrees of CCS dysfunction, including sinus bradycardia and AV block, even in the absence of overt structural heart disease.22 Analysis of Tbx5 transgenic mice demonstrated an exquisitely sensitive, dose-dependent correlation of Tbx5 expression level with severity of congenital heart defects and conduction disease.34 Interestingly, the temporal-spatial expression of Tbx5 correlates well with developmental timing of conduction system components.35 At E8.5, Tbx5 expression is enriched in a posterior-anterior axis, with highest levels of expression in the atria and sinus venosus, the site of SA node specification. During chamber formation, Tbx5 expression is graded in a left-right axis with highest levels in the left ventricle, particularly in the AV canal, ventricular conduction system and the trabecular myocardium (the site of Purkinje fiber development). Tbx5 expression is scant in the right ventricle except in the developing AV bundle, right bundle branch, and trabecular region.35 Both the dose–phenotype correlation and the temporospatial expression pattern of Tbx5 implicate it as a master regulator of conduction system development. However, the broad expression pattern of Tbx5 beyond the borders of the CCS, and the ability of Tbx5 with its binding partners, Nkx2-5 and Gata4, to elicit chamber myocardial gene programming suggests that it functions with CCS-restricted cofactors to elicit conduction specification.26,28 Numerous regulatory feedback loops have been identified in CCS components that enhance the conduction phenotype while simultaneously repressing chamber-specific gene programming (see Figure 29-4). These conduction-restricted cofactors will be discussed in their regional context. The mammalian sinoatrial node is a large comma-shaped structure with its head region located at the junction between the right superior vena cava and the right atrium, and the tail region situated along the crista terminalis. These regions represent distinct cellular lineages, as evidenced by their unique expression profiles. The SAN head, which constitutes up to 75% of SAN volume, develops from sinus venosus (SV) myocardium and retains the SV signature, Shox2+;Tbx3+;Tbx18+;Nkx2-5−.36,37 In contrast, working atrial myocytes derive from second heart field mesodermal progenitors and express Nkx2-5+;Shox2−;Tbx3−;Tbx18−. The SAN tail domain has an expression profile in between that of the head region and the atrial myocardium, expressing Shox2+;Tbx3+;Tbx18−;Nkx2-5weakly +. Lineage tracing studies have shown that the SAN tail originates from SV myocardium, but loses Tbx18 expression during development.37 Proper SAN development is dependent on the appropriate expression of Tbx5, Shox2, Tbx18, and Tbx3 (see Figure 29-4, B). Tbx5 expression in the sinus venosus is a critical regulator of the SAN signature through its actions on the transcriptional repressor, Tbx3, and the homeobox transcription factor, Shox2.34,38 Homozygous Tbx5del/del mice die embryonically at E10.5 because of severe hypoplasia of the sinoatrial region and of the primitive LV.27 Microarray analysis of Tbx5 heterozygous hearts, identified Tbx3 and Shox2 as significantly downregulated targets, and both factors showed reduced expression in the sinoatrial region.34,38 As stated earlier, Tbx3 is expressed throughout the developing and mature CCS (except the Purkinje network) and represses chamber-specific programming. Like Tbx5, Tbx3 displays critical dose dependency for proper differentiation and homeostatic maintenance of the conduction system.39 Analysis of Tbx3 mutant mice revealed a dose-dependent SAN phenotype with variable degrees of sinus node dysfunction and inappropriate expression of chamber-specific genes (Cx43, Cx40, Nppa, Scn5a) within the SAN region. Although the overall structure of the SAN is normal, SAN volume was reduced by approximately 45% to 60%, although this phenotype has not been consistently seen in all Tbx3 mutants when adjusted for weight.16,29,37,39 Ectopic overexpression of Tbx3 in atrial myocardium results in inappropriate suppression of chamber genes and upregulation of the SAN gene profile.29 These data suggest that Tbx3 is not essential for SAN formation, but is important for establishing and maintaining proper pacemaker gene programming. Shox2 is essential for formation of the sinoatrial valves and development of the sinoatrial node.40
Cell Biology of the Specialized Cardiac Conduction System
Histologic Analysis of the Developing Mammalian Cardiac Conduction System
Cellular Origins of the Cardiac Conduction System
Models of Cardiac Conduction System Development
Molecular Markers of the Cardiac Conduction System
Transcription Factor Regulatory Networks
The Sinoatrial Node
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cell Biology of the Specialized Cardiac Conduction System
