Cavopulmonary Shunts and the Hemi-Fontan Operation
Marshall L. Jacobs
Robert D. Stewart
HISTORY AND RATIONALE
The earliest palliative operations that were developed to treat various forms of congenital heart disease include the systemic-to-pulmonary artery shunt, first described by Blalock and Taussig in 1944, and the pulmonary artery band, first described in 1952 by Muller and Damman. In the earliest cases, these palliative procedures were not performed as elements of a planned approach that anticipated eventual definitive reconstruction, but rather in an attempt to prolong survival and improve the functional status of markedly compromised patients with either severe cyanosis or profound congestive heart failure. During the same era of innovation by pioneer cardiovascular surgeons, a third approach to palliation was explored by a number of investigators and surgical teams. This approach involved diverting some or all of the systemic venous return directly to the pulmonary arteries, bypassing the heart. The concept of cavopulmonary anastomosis may have had its origin in early studies of the concept of the “dispensable right ventricle.” Rodbard and Wagner in Chicago in 1949 had experimentally anastomosed the right atrial appendage to the pulmonary artery and ligated the main pulmonary artery in dogs, demonstrating the feasibility of excluding the right ventricle from the circulation. The first report of experimental cavopulmonary connection was published in 1950 in Italy by Carlo Carlon of Padua who hypothesized that an “advantage would be received if the blood of the superior vena cava (SVC) should reach the capillary region of the right lung by way of a convenient anastomosis between the great venous trunk and the arterial system of the right lung.” Carlon’s canine preparation was an end-to-end anastomosis between the proximal end of the divided azygous vein and the right pulmonary artery with pre-atrial ligation of the superior vena cava. In 1951, in the first English language report of these experiments, Carlon and associates wrote, “We are not aware that anyone else has foreseen and studied the problem of oxygenation of the pulmonary blood under venous pressure and without cardiac output.” Carlon’s first clinical experience was not reported until 1964. In the meantime, Glenn and Patino at Yale published their first report of experimental cavopulmonary shunts in 1954, and in 1955 reported a large study of 59 operated dogs, with six long-term survivors. Glenn’s experimental preparations explored the feasibility and physiology of both superior cavopulmonary connections and inferior cavopulmonary connections, noting the occurrence of chylothorax in some instances of the former strategy and ascites in the latter. The first clinical report by Glenn was published in 1958. It is following that report, and a subsequent series of publications by the same authors, that the superior cavopulmonary anastomosis has become widely known as the “Glenn shunt.” Interestingly, however, a fundamentally similar operation was at the same time being developed and evaluated in Budapest by Frances Robiscek and associates, in Russia by Galankin and Darbinian and by Meshalkin, in the United States by Shumacher, and in Italy by Carlon and others, as stated above. In retrospect, it appears likely that the first attempts at clinical cavopulmonary shunts were by Harris B. Shumacher in 1954. His two young patients, one with truncus arteriosus and one with transposition, both had markedly elevated pulmonary vascular resistance and both died within hours of operation.
The “Glenn shunt” (unidirectional superior cavopulmonary anastomosis) found a unique and important place in the management of cyanotic heart disease in general, and functionally univentricular hearts in particular. The resulting physiology was fundamentally different from that achieved by creation of systemic-to-pulmonary artery shunts. The cavopulmonary anastomosis was capable of increasing pulmonary blood flow and thus systemic arterial oxygen saturation without increasing the volume load on the systemic ventricle. It also proved the feasibility of transpulmonary flow without complete dependence on a subpulmonary ventricle. During the 1960s and early 1970s, the classic “Glenn shunt” was used extensively, with good results, to palliate patients with tricuspid atresia, and to a lesser extent other forms of functionally single ventricle. Unfortunately, it became apparent after a number of years that cyanosis would eventually recur in a high proportion of patients. This was in part attributable to maldistribution of blood flow in the involved lung (favoring flow to the lower lobe), and in part to the development of arteriovenous shunting within the lung that was perfused exclusively with superior vena caval blood. The “unidirectional” Glenn shunt was eventually modified in a variety of ways by Dogliotti, Haller, and Azzolina to allow the flow of superior vena caval blood into both pulmonary arteries. They utilized either an end-to-side or side-to-side anastomosis of the superior vena cava to the right pulmonary artery with maintenance of continuity between right and left pulmonary artery branches. This technique, which preserves the confluence and the integrity of the central pulmonary arteries, eventually supplanted the classic Glenn anastomosis as preferred palliation for functional single ventricles. Currently, without particularly good reason, the bidirectional superior cavopulmonary anastomosis is generally and widely referred to as the “bidirectional Glenn shunt.” It is most widely used as a preliminary or interim procedure (following neonatal palliation) in patients with functionally univentricular hearts for whom a staged approach to reconstruction culminating in completion of the Fontan circulation is anticipated. Occasionally, construction of a superior cavopulmonary
connection, together with maintenance of a physiologically restrictive source of antegrade pulmonary blood flow from the functionally univentricular heart or from a small patent arterial duct or shunt, is considered as a final or definitive palliative arrangement, as an alternative to the Fontan procedure. Other applications of the bidirectional superior cavopulmonary connection are less common. They include those circumstances where mild-to-moderate degrees of hypoplasia of the right ventricle and its associated valves permit the use of the right ventricle as a subpulmonary ventricle that handles venous return from the inferior vena cava, while superior caval return is diverted directly to the lungs. This arrangement has come to be known colloquially as “one-and-a-half ventricle repair.” Occasionally, in the setting of complex malformations with atrioventricular discordance, the superior cavopulmonary connection has been used in conjunction with a simplified atrial baffle procedure that deals only with inferior caval return. Finally, superior cavopulmonary connection has, in rare instances, been used to achieve right ventricular volume unloading in the setting of ischemic right ventricular failure. It has been suggested that it may have an analogous role in patients with reduced right ventricular function who are candidates for left ventricular mechanical support.
connection, together with maintenance of a physiologically restrictive source of antegrade pulmonary blood flow from the functionally univentricular heart or from a small patent arterial duct or shunt, is considered as a final or definitive palliative arrangement, as an alternative to the Fontan procedure. Other applications of the bidirectional superior cavopulmonary connection are less common. They include those circumstances where mild-to-moderate degrees of hypoplasia of the right ventricle and its associated valves permit the use of the right ventricle as a subpulmonary ventricle that handles venous return from the inferior vena cava, while superior caval return is diverted directly to the lungs. This arrangement has come to be known colloquially as “one-and-a-half ventricle repair.” Occasionally, in the setting of complex malformations with atrioventricular discordance, the superior cavopulmonary connection has been used in conjunction with a simplified atrial baffle procedure that deals only with inferior caval return. Finally, superior cavopulmonary connection has, in rare instances, been used to achieve right ventricular volume unloading in the setting of ischemic right ventricular failure. It has been suggested that it may have an analogous role in patients with reduced right ventricular function who are candidates for left ventricular mechanical support.
ROLE OF THE SUPERIOR CAVOPULMONARY CONNECTION IN THE MANAGEMENT OF FUNCTIONALLY UNIVENTRICULAR HEARTS
By the 1980s, refinement of the technical aspects of modified Fontan operations and development of guidelines for patient selection led to a decrement in the overall mortality associated with these procedures. Nonetheless, a troublesome degree of morbidity and early mortality occurred following modified Fontan operations, even among those who met all criteria for “favorable candidate” status. Too often, even among carefully selected patients with adequate preoperative ventricular function and pulmonary vascular anatomy and resistance, the postoperative course was characterized by elevation of central venous pressure, tachycardia, with sequestration of fluid in the pleural and peritoneal spaces, and hypotension which was only transiently responsive to volume administration at the expense of further increases in venous pressure. Little improvement was seen with administration of inotropic agents and vasodilators, or with external compressive devices to promote venous return while minimizing volume administration. Echocardiography frequently showed a striking degree of ventricular wall thickness relative to cavity volume, vastly out of proportion to the preoperative degree of myocardial hypertrophy. This appearance of a thickened ventricle with relatively small cavity volume was associated with tachycardia and poor perfusion, regardless of the contractile state of the myocardium. It became evident that the conversion from the unoperated or palliated state to the post-Fontan circulation was associated with rapid removal of a chronic volume load on the ventricle. The change in loading conditions took place abruptly, while regression of myocardial mass proceeds slowly. As a consequence, a maladaptive response to removal of the ventricular volume load occurs, characterized by markedly increased ventricular myocardial mass-to-volume ratio. The persistence of increased muscle mass in the setting of acutely diminished ventricular volume results in increased ventricular wall thickness and decreased cavity dimensions. This change in the geometry of the ventricle can result in significant alterations in both systolic and diastolic functions. Perhaps most importantly, the diminished compliance of the acutely thickened ventricle results in poor filling, at the expense of elevated pressure in the cavopulmonary pathway and diminished cardiac output. This realization shed light on earlier observations that older age (longer duration of volume loading) and increased ventricular hypertrophy (increased muscle mass) were risk factors for mortality in association with the Fontan operation. More importantly, it leads to the hypothesis that dividing Fontan’s operation into two procedures could accomplish early reduction of the volume work of the single ventricle, at the same time minimizing the impact of changes in ventricular geometry on outcome and survival. Following construction of a superior cavopulmonary connection and elimination of all other sources of pulmonary blood flow (the first stage), a gradual adaptive process characterized by normalization of the mass-to-volume relationship of the functionally single ventricle would make it possible to perform the completion Fontan procedure (the second stage) with the expectation of minimal change in ventricular geometry and favorable conditions with regard to the ventricle’s diastolic properties (compliance).
At the end of the decade of the 1980s, and in the early 1990s, several groups explored the potential benefits of a two-staged approach to Fontan operation. For some, the protocol of performance of an early bidirectional superior caval pulmonary anastomosis followed later by a completion Fontan procedure was a pathway selected specifically for high-risk Fontan candidates. Others, notably Norwood and Jacobs in Philadelphia, pursued a two-staged approach to Fontan operation for virtually all patients. They postulated that some of the morbidity and mortality associated with Fontan operation would be eliminated by staging. Performance of a superior cavopulmonary connection in the first year of life achieved construction of part of the Fontan pathway. More importantly, the duration of the palliated state was minimized, and the volume load on the single ventricle was reduced as early in life as is practical. Thus in 1989, Norwood introduced the hemi-Fontan procedure, as the first step in a two-stage process of achieving total cavopulmonary connection. Obligating superior vena caval return to pass through the lungs before returning to the functional single ventricle, the hemi-Fontan operation is the physiologic equivalent of the bidirectional Glenn anastomosis. Because it is planned as an intermediate step before an anticipated completion Fontan procedure, it differs technically from a bidirectional Glenn anastomosis in ways that simplify the eventual completion Fontan.
INDICATIONS AND PREOPERATIVE ASSESSMENT
The most common indication for a cavopulmonary shunt is anatomy that is not amenable to eventual biventricular repair as a means of achieving systemic and pulmonary circulations in series. Superior cavopulmonary connections are generally considered as a preparatory stage for any unoperated or palliated patient older than about 3 months of age in which a univentricular repair is anticipated, particularly for those patients with risk factors for a Fontan-type procedure. In these situations, a cavopulmonary connection may be combined with a procedure to address factors that may increase the risk of Fontan operation, such as systemic ventricular outflow tract obstruction, atrioventricular valve (AV) insufficiency, excessive ventricular volume load associated with systemic-to-pulmonary artery shunts, impediments to pulmonary venous return, or pulmonary artery distortion. It may also be a rational
staging procedure for selected patients for whom the ultimate choice between univentricular and biventricular repair is not clear. Finally, some patients with functionally univentricular hearts may be destined for heart transplantation because of either poor myocardial function or severe organic systemic AV valve abnormalities. In these rare instances, reduction of the volume burden on the ventricle by means of superior cavopulmonary connection may stabilize the physiology while awaiting transplantation and may even result in improved ventricular function and/or lessened AV valve regurgitation.
staging procedure for selected patients for whom the ultimate choice between univentricular and biventricular repair is not clear. Finally, some patients with functionally univentricular hearts may be destined for heart transplantation because of either poor myocardial function or severe organic systemic AV valve abnormalities. In these rare instances, reduction of the volume burden on the ventricle by means of superior cavopulmonary connection may stabilize the physiology while awaiting transplantation and may even result in improved ventricular function and/or lessened AV valve regurgitation.
The cavopulmonary connection has several advantages as an interim procedure in preparation for an eventual completion Fontan procedure. First, it can be performed with a lower mortality and morbidity than a Fontan procedure or a total cavopulmonary connection, particularly in infants from 3 to 12 months of age. Second, in contrast to a systemic-to-pulmonary artery shunt, a cavopulmonary shunt increases effective pulmonary blood flow by directing fully desaturated blood into the pulmonary circulation. Third, the risk of developing pulmonary vascular disease is reduced by lowering pulmonary arteriolar pressure. Fourth, the risk of pulmonary artery distortion is less than with a systemic-to-pulmonary artery shunt. Finally, and perhaps most important, the cavopulmonary shunt, when combined with removing systemic-to-pulmonary artery shunts or antegrade pulmonary blood flow, reduces ventricular work by reducing the volume load on the single ventricle. As noted above, this often improves ventricular function and reduces systemic AV valve regurgitation, which would compromise candidacy for a Fontan procedure.
Careful assessment by echocardiography is undertaken as part of the preoperative planning prior to surgery to create cavopulmonary connections. Comprehensive evaluation of the anatomy includes assessment of the segmental relations and connections, the morphology and function of the ventricle(s), presence and degree of AV regurgitation, presence and degree of systemic ventricular outflow tract obstruction (including aortic arch obstruction), and the nature of systemic and pulmonary venous connections. In particular, the existence of bilateral superior caval veins and/or the possibility of interrupted inferior vena cava with azygos continuation must be considered. All potential sources of pulmonary blood flow (antegrade from the heart, patent ductus, surgically created shunt(s), aortopulmonary collaterals) should be evaluated and the architecture of the intrapericardial pulmonary arteries must be assessed. Cardiac catheterization is indicated when these details cannot be reliably assessed by means of echocardiography or cardiac magnetic resonance imaging, or when a physiologic assessment is critical to surgical decision making or risk stratification. Determination of pulmonary vascular resistance may be important in the uncommon circumstance presented by an operated or palliated older patient. Measurement of pulmonary venous wedge pressure is generally informative if direct measurement of pulmonary artery pressure and pulmonary blood flow is not easily accomplished. Occasionally, imaging studies will suggest the presence of abnormalities of venous anatomy that may best be managed by catheter-directed intervention prior to surgery. An example would be venovenous collateral connections from systemic veins to pulmonary veins, or the presence of a small secondary (usually leftsided) superior vena cava or cardinal vein, which is considered too small to be effectively incorporated into bilateral superior cavopulmonary connections.
Significant pulmonary artery distortion or stenosis should be identified during preoperative evaluation so that it can be addressed as part of the cavopulmonary shunt operation. Some centers rely on determination of pulmonary artery size prior to performance of cavopulmonary connection. We do not generally exclude patients from superior cavopulmonary connection on the basis of hypoplasia of the intrapericardial pulmonary artery branches. Superior cavopulmonary anastomosis has been accomplished successfully in patients with Nakata indices as low as 70 mm2/m2.
OPERATIVE TECHNIQUE
The Unidirectional Superior Cavopulmonary Anastomosis (“Classic Glenn Shunt”)
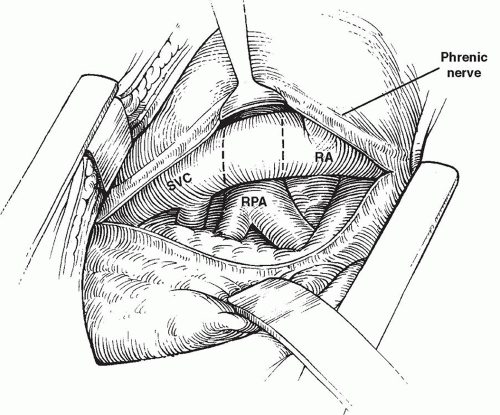
The “unidirectional” or “classic Glenn” cavopulmonary shunt is largely of historical interest and is rarely performed in the current era. Typically, the procedure was undertaken without cardiopulmonary bypass support. The surgical approach was most often a posterolateral thoracotomy incision through the fourth intercostal space. Once this exposure has been accomplished, the pleura and pericardium are incised posterior to the phrenic nerve, which is left attached to the widely mobilized anterior pleuropericardium to avoid excessive traction directly on the nerve (Fig. 94.1). The superior vena cava is dissected free from the level of the
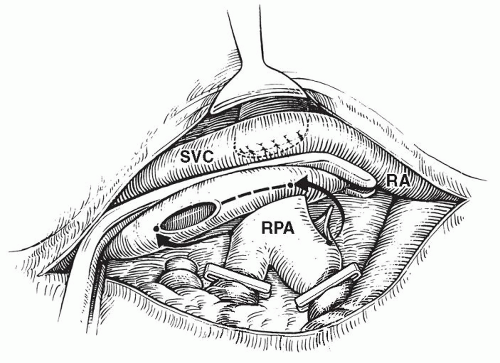
innominate vein to the right atrium. The azygos vein is ligated and divided to provide better mobility of the superior vena cava and to prevent collateral venous connection with the inferior vena cava. The right pulmonary artery is dissected from its central origin to beyond the first hilar branches. After intravenous administration of heparin, the proximal right pulmonary artery is occluded with a vascular clamp. Oxygen saturation at this time is dependent entirely on flow to the contralateral lung. If oxygenation and hemodynamics are acceptable in the face of this test occlusion of the right pulmonary artery, the distal branches of the right pulmonary artery are occluded with silastic vessel loops or small atraumatic clamps. The right pulmonary artery is then divided at a point medial to the superior vena cava, the proximal end is oversewn, and the clamp is removed. The orifice of the right pulmonary artery may be enlarged slightly by extending the opening inferiorly. A partially occluding (side-biting) vascular clamp is placed on the superior vena cava from above the divided azygos vein to just above the right atrium, isolating a long segment of vena cava (Fig. 94.2). Careful placement of the clamp avoids complete obstruction of superior vena caval drainage to the right atrium. An incision is made in the lateral aspect of the superior vena cava, beginning where the inferior margin of the right pulmonary artery crosses the vena cava and extending superiorly through the orifice of the divided azygos vein. End-to-side anastomosis of the right pulmonary artery to the superior vena cava is then accomplished using fine monofilament suture. Care is taken to avoid “purse stringing,” including the placement of a few interrupted sutures. Distal vessel loops are released, followed by removal of the side-biting clamp from the superior vena cava (Fig. 94.3). Following a period of manual ventilation to fully inflate the right lung, the superior vena cava is temporarily occluded above its junction with the right atrium. It is customary to make a direct measurement of pressure in the superior vena cava at this point, before proceeding with definitive ligation of the superior vena cava above its junction with the right atrium. An alternative surgical method of creating a unidirectional superior cavopulmonary anastomosis involves transection of the superior vena cava above its junction with the right atrium and direct end-to-end anastomosis of the cava to the divided right pulmonary artery. This approach may be facilitated either by the use of a decompressing shunt from the innominate vein to the right atrium or by a brief period of cardiopulmonary bypass support.
innominate vein to the right atrium. The azygos vein is ligated and divided to provide better mobility of the superior vena cava and to prevent collateral venous connection with the inferior vena cava. The right pulmonary artery is dissected from its central origin to beyond the first hilar branches. After intravenous administration of heparin, the proximal right pulmonary artery is occluded with a vascular clamp. Oxygen saturation at this time is dependent entirely on flow to the contralateral lung. If oxygenation and hemodynamics are acceptable in the face of this test occlusion of the right pulmonary artery, the distal branches of the right pulmonary artery are occluded with silastic vessel loops or small atraumatic clamps. The right pulmonary artery is then divided at a point medial to the superior vena cava, the proximal end is oversewn, and the clamp is removed. The orifice of the right pulmonary artery may be enlarged slightly by extending the opening inferiorly. A partially occluding (side-biting) vascular clamp is placed on the superior vena cava from above the divided azygos vein to just above the right atrium, isolating a long segment of vena cava (Fig. 94.2). Careful placement of the clamp avoids complete obstruction of superior vena caval drainage to the right atrium. An incision is made in the lateral aspect of the superior vena cava, beginning where the inferior margin of the right pulmonary artery crosses the vena cava and extending superiorly through the orifice of the divided azygos vein. End-to-side anastomosis of the right pulmonary artery to the superior vena cava is then accomplished using fine monofilament suture. Care is taken to avoid “purse stringing,” including the placement of a few interrupted sutures. Distal vessel loops are released, followed by removal of the side-biting clamp from the superior vena cava (Fig. 94.3). Following a period of manual ventilation to fully inflate the right lung, the superior vena cava is temporarily occluded above its junction with the right atrium. It is customary to make a direct measurement of pressure in the superior vena cava at this point, before proceeding with definitive ligation of the superior vena cava above its junction with the right atrium. An alternative surgical method of creating a unidirectional superior cavopulmonary anastomosis involves transection of the superior vena cava above its junction with the right atrium and direct end-to-end anastomosis of the cava to the divided right pulmonary artery. This approach may be facilitated either by the use of a decompressing shunt from the innominate vein to the right atrium or by a brief period of cardiopulmonary bypass support.
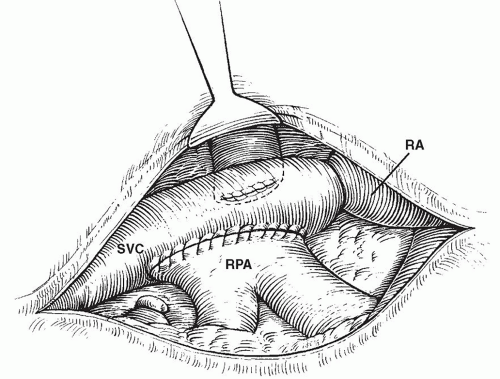 Fig. 94.3. A completed “classic Glenn” cavopulmonary shunt with ligation of the lower superior vena cava (SVC). RA, right atrium; RPA, right pulmonary artery. |
The unidirectional Glenn anastomosis involves iatrogenic disruption of continuity between the right and left pulmonary arteries, which is viewed as disadvantageous in patients for whom eventual Fontan circulation is anticipated. A rare circumstance where the unidirectional cavopulmonary anastomosis might be performed today would be in a patient with an isolated or very hypoplastic left pulmonary artery that is being “resuscitated” with a systemic-to-pulmonary artery shunt. Superior vena caval flow may be entirely diverted to the right pulmonary artery in order to achieve reduction of the volume load on the functionally single ventricle. In general, awareness of technical issues related to the unidirectional Glenn shunt is primarily of importance today because of the likelihood of encountering older patients who underwent such palliative procedures in the past and the need to reestablish pulmonary artery continuity in the context of a Fontan procedure, a Fontan conversion, or even heart transplantation.
The Bidirectional Superior Cavopulmonary Anastomosis (“Bidirectional Glenn”)
The bidirectional cavopulmonary anastomosis is most frequently constructed as part of the staged palliation of functionally univentricular heart disease in a patient for whom eventual completion of the Fontan circulation by means of an extracardiac conduit type of completion Fontan procedure is anticipated. Median sternotomy is by far the most common surgical approach. Cardiopulmonary bypass support is used in the majority of instances. Cardiopulmonary bypass can be avoided selectively, in cases where no intracardiac procedures are required, as long as blood flow to the contralateral lung is sufficient to maintain adequate systemic arterial saturation during clamping of the ipsilateral branch pulmonary artery, and adequate cerebral blood flow is maintained with the avoidance of severe cerebral venous hypertension. In general, this involves the use of a temporary shunt that diverts blood from the superior vena cava to the right atrium. This is accomplished under conditions of systemic heparinization, by the placement of a cannula in the innominate vein and another in the right atrium, and connecting them. The use of cardiopulmonary bypass support is required if total occlusion of the ipsilateral proximal pulmonary artery results in an unacceptable degree of systemic arterial desaturation, if an ipsilateral aortopulmonary shunt is present and is the principle source of pulmonary blood flow, if superior vena cava pressure is too high following caval occlusion despite the presence of the cava to right atrium shunt, or if cerebral oxygen saturation falls to an unsafe level. Cardiopulmonary bypass is required, of course, if concomitant intracardiac procedures are anticipated. Many, but not all surgeons, prefer to routinely use cardiopulmonary bypass support for cavopulmonary shunt operations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree