Chapter 94 Catheter Ablation for Atrial Fibrillation
Clinical Techniques, Indications, and Outcomes
The prevalence of atrial fibrillation (AF) in Western countries continues to rise, with 30 million patients estimated to be affected by 2050 across the United States and Europe alone.1 Catheter ablation of AF is currently an established treatment to achieve cure in a substantial proportion of patients.2 Multiple randomized clinical trials have demonstrated a clear superiority of catheter ablation over antiarrhythmic drug therapy to achieve lasting sinus rhythm maintenance, improve symptoms and quality of life, and possibly reverse the AF-associated risk of thromboembolic complications.3–5
To achieve such important outcomes and develop highly effective ablation techniques, intense research has been directed toward the discovery and validation of electrophysiological and anatomic targets fundamental for triggering and maintaining the arrhythmia.6–10
After the pivotal demonstration that focal discharges from the pulmonary veins (PVs) are implicated in the initiation of AF, empirical PV isolation has been performed with the highest procedural success in patients with paroxysmal AF, in whom spontaneous PV firing is frequently the only trigger for AF paroxysms.4,9,11 Patients with AF of longer duration, such as those with persistent and longstanding persistent AF, may require targeting additional sites for successful ablation, including the entire left atrial posterior wall and complex fractionated atrial electrograms (CFAE).12,13 Moreover, targeting other sites of AF initiation has been demonstrated to offer incremental benefit to patients presenting for repeat ablation procedures.14
Patient Selection and Pre-procedural Management
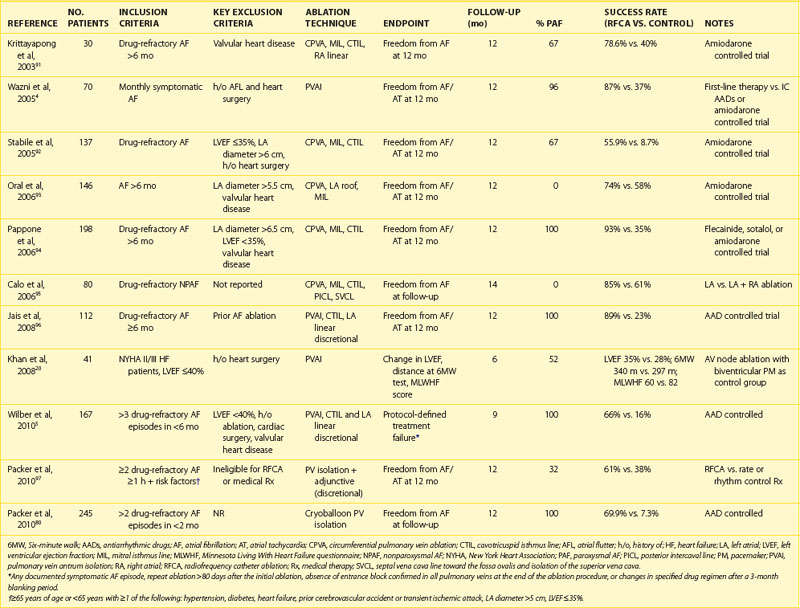
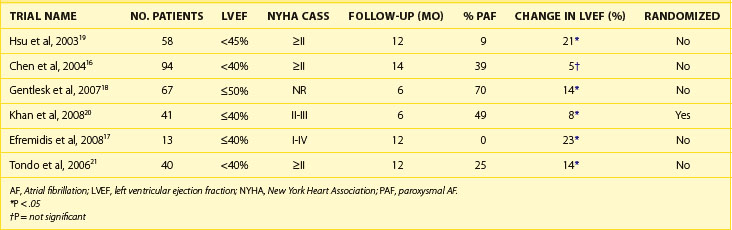
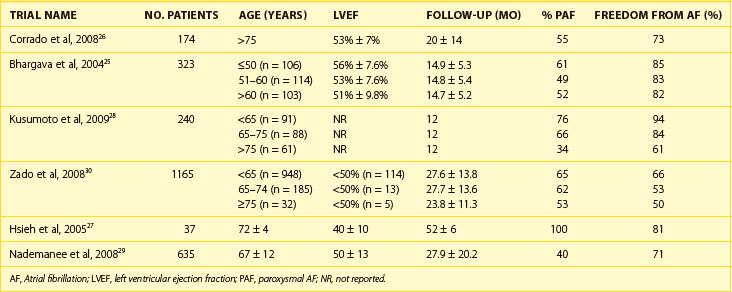
The 2011 edition of the American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) guidelines for the management of AF gives a class I indication with the highest level of evidence to catheter ablation in patients with symptomatic paroxysmal AF who have not responded to treatment with an antiarrhythmic drug.15 Of note, updated guidelines have also introduced new recommendations for catheter ablation in patients with persistent AF, significant left atrial dilation, and left ventricular dysfunction (Tables 94-1 to 94-3).15–21 Evidence suggests that catheter ablation is successful in patients not yet included in guideline recommendations, such as those with previous cardiac surgery or valvular heart disease, and subgroups such as older adults and women (Table 94-4).22–30 Accordingly, catheter ablation should be offered to nearly all patients with symptomatic drug-refractory AF.
Table 94-1 Current Recommendations for Catheter Ablation of Atrial Fibrillation
| CLINICAL SCENARIO | CLASS OF RECOMMENDATION | LEVEL OF EVIDENCE |
|---|---|---|
| Symptomatic paroxysmal AF, failed treatment with an AAD | I | A |
| Symptomatic persistent AF | IIa | A |
| Symptomatic paroxysmal AF, significant left atrial dilation or LV dysfunction | IIb | A |
AAD, Antiarrhythmic drug; AF, atrial fibrillation; LV, left ventricular.
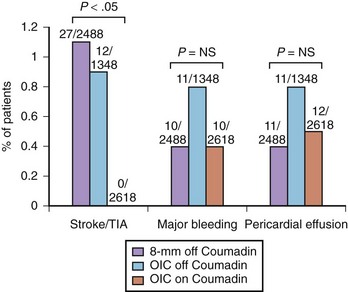
This approach has been demonstrated to decrease peri-procedural complications compared with warfarin discontinuation and bridging with heparin.31 In a large cohort study, including 6454 patients undergoing ablation in nine different centers, 2488 underwent ablation with an 8-mm ablation catheter and pre-procedural warfarin discontinuation (group 1), 1348 underwent ablation with an open irrigated catheter and pre-procedural warfarin discontinuation (group 2), and 2618 underwent ablation with an open irrigated catheter without pre-procedural warfarin discontinuation (group 3). Overall, peri-procedural thromboembolic complications occurred in 39 (0.6%) patients, with a rate of 1.1% in group 1 and 0.9% in group 2. Of note, none in group 3 had peri-procedural thromboembolism (Figure 94-1). These data also support the finding that pre-procedural TEE does not add much if therapeutic INR is present on the day of the procedure and has been verified every week in the month preceding the ablation.
Importantly, the anticoagulation strategy of ablation with a therapeutic INR was confirmed to be a strong and independent predictor of lower peri-procedural thromboembolic events at multivariable analysis (odds ratio, 0.54; 95% confidence interval, 0.32 to 0.89; P = .017). With regard to bleeding, the pooled rate of major bleeding complications (e.g., bleeding requiring interventions, including transfusions, hemopericardium, hemothorax, and retroperitoneal bleeding) and pericardial effusion in patients who discontinued warfarin before the ablation procedure (groups 1 and 2) was 1.1%, whereas in group 3 it was 0.8% (see Figure 94-1).31
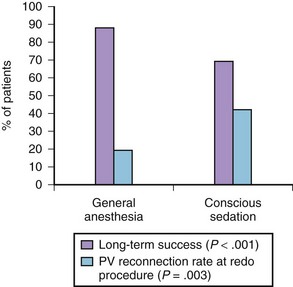
The anesthesia protocol adopted during ablation can affect the procedural results. In a randomized trial, 257 consecutive patients undergoing the first AF ablation were allocated to general anesthesia (n = 129) or conscious sedation (n = 128).32 General anesthesia was initiated with propofol (2 mg/kg) and fentanyl (1 to 2 µg/kg), followed by a neuromuscular blocking agent (usually rocuronium 0.6 to 1 mg/kg) and by endotracheal intubation, with intermittent positive-pressure ventilation. Conscious sedation was obtained with fentanyl or midazolam. At 17 ± 8 months of follow-up, 88 (69%) patients assigned to conscious sedation were free of atrial arrhythmias off antiarrhythmic drugs compared with 114 (88%) patients randomized to general anesthesia (P < .001) (Figure 94-2). All patients with recurrence had a second procedure. Interestingly, at the repeat procedure, 42% of PVs in the conscious sedation arm had recovered PV conduction compared with 19% in the general anesthesia group (P = .003).32 Therefore current evidence supports the adoption of general anesthesia or other strategies to achieve better catheter stability, such as jet ventilation.33
Techniques and Results of Ablation in Paroxysmal Atrial Fibrillation
Pulmonary Vein Isolation
PVs are the trigger site for paroxysmal AF in the majority of patients. Over the years, catheter ablation of PV triggers has undergone a profound evolution. Initial attempts at focal PV ablation involved longitudinal mapping of triggers of AF in the PVs and focal ablation.11,34,35 The clinical outcome of such an approach, which often required multiple electrical cardioversions during the ablation procedure because of the necessity of repeat induction of AF for mapping, was fairly disappointing.11,34,35 Moreover, radiofrequency (RF) delivery within the PVs for focal ablation increased the risk of PV stenosis.36,37
The procedure evolved to target the ostium of the tubular portion of the PVs to isolate electrically the myocardial connections between the PV and the left atrium. This procedure is commonly referred to as segmental ostial ablation.6 Also, with segmental ostial ablation, initial attempts were aimed at ablating only the PVs that had evidence of arrhythmogenic activity. However, it became rapidly appreciated that empirical isolation of all PVs was necessary to increase the procedural success because more than 85% of patients actually have multiple arrhythmogenic PVs.
Therefore subsequent evolution of PV ablation involved further movement downstream in the atrium, targeting the so-called PV antrum.8,37,38 To achieve PV antrum isolation (PVAI), multiple approaches with different mapping systems have been described. These include electroanatomic mapping using three-dimensional nonfluoroscopic systems and circular mapping techniques guided by imaging the PVs through intracardiac echocardiography (ICE) or angiography.8,9,37–40
Our current approach consists of PVAI guided by a circular mapping catheter and ICE. This approach has been demonstrated to be superior to other ablation techniques in studies of direct comparison and is one of the most reproducible and standardized techniques to achieve PVAI because it has reproducible effectiveness in different institutions and with different operators (Figures 94-3 and 94-4).2,38,41,42
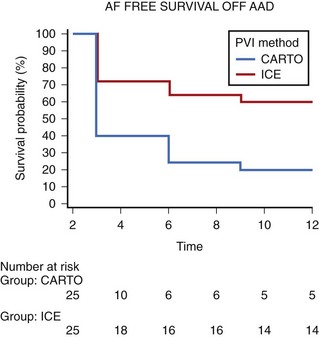
In particular, our experience with circumferential PV ablation guided by nonfluoroscopic three-dimensional mapping systems (CARTO, Biosense Webster, Diamond Bar, CA) has been disappointing.37,41 In a previous study, we evaluated such an approach in 71 patients with early recurrence of AF after electrical cardioversion. After a mean follow-up of 29 months, 17% of patients had AF recurrences refractory to antiarrhythmic drug therapy, and 62% remained on antiarrhythmic drugs. These suboptimal results were associated with a rate of PV stenosis of 36%, which was severe in nearly one third of cases, and with a rate of PV reconnection of 69%.37 Recently, we reported the results of a randomized direct comparison between CARTO-guided circumferential PV ablation and PVAI using ICE and a circular mapping catheter.41 Overall, 60 patients with drug-refractory AF underwent randomization. The endpoint assessed was long-term procedural success, defined as absence of atrial tachyarrhythmias off antiarrhythmic drugs. The mean procedural time was comparable between the two approaches, although PVAI was associated with longer fluoroscopy time. At a mean follow-up of 24 ± 12 months, PVAI was more likely to achieve control of atrial tachyarrhythmias off antiarrhythmic drugs (57% vs. 27%, P = .02) (Figure 94-5).41
The superiority of PVAI guided by circular mapping catheter over electroanatomic mapping–guided circumferential PV ablation has been confirmed by other groups.43 Karch et al conducted a randomized study on 100 patients, comparing circumferential PV ablation and segmental PVAI guided by a circular mapping catheter.43 In that study, PVAI guided by circular mapping catheter was associated with shorter procedural time compared with circumferential PV ablation (256 ± 72 min vs. 284 vs. 86 min; P = .02), although the fluoroscopy time with a circular mapping–guided approach was confirmed to be longer (72 ± 26 min vs. 45 ± 21 min, respectively; P < .01). At 6-month follow-up, 42% of patients allocated to circumferential PV ablation and 66% of those receiving PVAI were free of atrial tachyarrhythmia episodes (P = .02 for comparison).
Putting together these results, electroanatomic mapping–guided circumferential PV ablation appears unreliable in completely isolating the PVs, which supports a circular mapping–guided technique to achieve effective PV ablation. The latter approach also offers the potential advantages of shortening the procedural time, limiting the delivery of RF energy to discrete areas showing the earliest PV potentials.38,40 Moreover, because with circumferential PV ablation, contiguous lesions are created without necessarily achieving complete conduction block, post-incisional re-entrant atrial tachycardia (AT) has been reported with this approach in up to 20% of patients.44
As far as techniques for PVs imaging are concerned, ICE has been demonstrated to be of incremental value compared with other techniques such as PV angiography.9 We have reported the outcome of PVAI in 315 patients: 56 underwent ablation-guided by circular mapping catheter and PV angiography (group 1), 107 patients underwent circular mapping catheter and ICE-guided ablation (group 2), and 152 patients received circular mapping–guided ablation with titration of RF energy based on visualization of microbubbles by ICE (group 3). After a mean follow-up of 13 ± 4 months, 19.6% of patients in group 1 had AF recurrence compared with 16.8% of those in group 2 and 9.8% in group 3 (P < .01 vs. group 1). Fluoroscopy and procedure times were significantly lower in groups 2 and 3 compared with group 1, and no cases of severe PV stenosis were observed in group 3 patients compared with a rate of 3.5% in group 1 and 1.8% in group 2.9
Adjunctive Non–Pulmonary Vein Targets
As an adjunct to PVAI, patients in our institution undergo systematic isolation of the superior vena cava (SVC) as well. This approach is supported by robust clinical evidence.45,46 In a randomized study of 320 consecutive patients with AF (46% paroxysmal, 23% persistent, 31% permanent), we assessed the incremental value of systematic isolation of the SVC as an adjunctive strategy to PVAI versus PVAI alone. At 12-month follow-up, a significant difference in procedural success between the two approaches was found solely in patients presenting with paroxysmal AF (77% in the PVAI alone vs. 90% in the PVAI plus isolation of the superior vena cava; P = .04 for comparison).46 The technique adopted for SVC isolation is similar to that for PVAI—a circular mapping catheter–guided technique with ICE assistance. Caution should be exercised when isolating the lateral portion of the SVC because of its proximity to the right phrenic nerve. In this case, high-voltage pacing (>30 mA) is used to check for phrenic nerve stimulation before delivering RF energy.
Extending ablation beyond the PV antra and SVC in patients with paroxysmal AF is unnecessary.42 In a recent randomized study, we aimed to assess whether adding bi-atrial ablation of the CFAE to PVAI in patients with paroxysmal AF presenting in the electrophysiology laboratory improved the procedural success compared with PVAI alone. At a follow-up of 1 year, no difference in terms of success rates was found between PVAI alone and PVAI plus ablation of CFAE (89% vs. 91%).42
Techniques and Results of Ablation in Persistent and Longstanding Persistent Atrial Fibrillation
Current guidelines define persistent AF as either lasting longer than 7 days or requiring termination by cardioversion. Persistent AF is defined as longstanding when it has lasted for 1 year or longer despite cardioversion and antiarrhythmic therapy.15
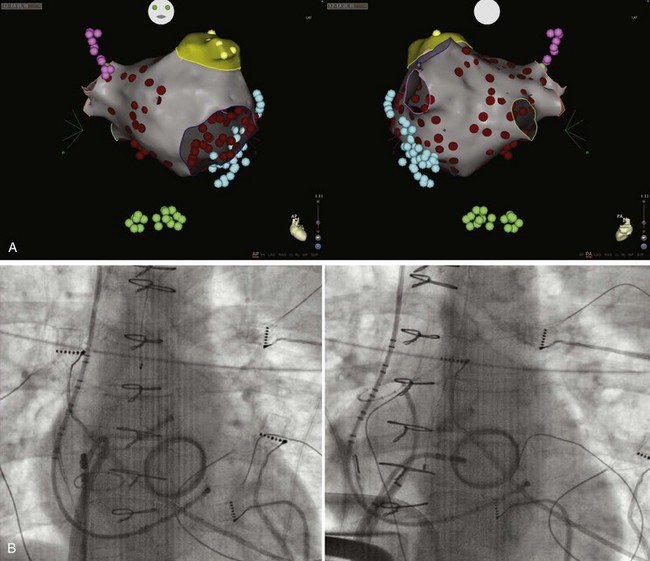
In patients with persistent AF, as an adjunct to PVAI and SVC isolation, we extend ablation to the entire left atrial posterior wall down to the coronary sinus and the entire left side of the septum (Figure 94-6). CFAE in the left atrial chamber and within the coronary sinus are targeted as well.12,47 This approach is well supported by available evidence.48
Isolation of the Left Atrial Posterior Wall
From an embryologic, anatomic, and electrophysiological standpoint, the posterior left atrial wall should be considered an extension of the PVs (Figure 94-7).49 Rotors and high-frequency sources of AF have been demonstrated within the posterior left atrial wall in preclinical studies, and sentinel observations from intraoperative AF ablation have confirmed a significant role of the posterior wall for triggering and maintaining the arrhythmia.50 In this regard, AF localized to the posterior wall and dissociated from sinus rhythm has been reported after en bloc encircling of all PVs and the posterior wall.51,52
In patients with persistent and longstanding persistent AF, significant changes in atrial structure and electrophysiological function (i.e., atrial remodeling) further increase the chance that non-PV sites are involved in triggering and maintaining the arrhythmia. This supports the inadequacy of PV isolation alone as an effective ablation strategy.12,13,47,53,54 Ablation of the posterior wall has been suggested to be of equal effectiveness compared with circumferential PV ablation in patients with chronic AF. In a randomized trial, 80 patients with chronic AF were assigned to circumferential PV ablation or to non-encircling linear ablation of the posterior wall. At a mean follow-up of 9 months, AF recurred in 28% of patients who underwent circumferential PV ablation compared with 25% of those who received posterior wall linear ablation (P = .8 for comparison).55
Substrate-Modifying Approaches: Ablation of Complex Fractionated Atrial Electrograms
The concept of targeting the left atrial electrophysiological substrate to cure AF derives from early surgical experience on AF mapping, which demonstrated critical sites of slow and discontinuous conduction characterized by strikingly fractionated atrial electrograms.56
Endocardial mapping and ablation of areas displaying CFAEs as a curative approach for AF has been firstly described by Nademanee et al.13 In their original contribution, CFAE were defined by visual criteria as low-amplitude potentials (0.06 to 0.25 mV) with consistent temporal and spatial stability and either fractionated atrial electrograms (composed by two or more deflections) or atrial electrograms with cycle length of 120 ms or greater. Applying a substrate-based approach targeting only areas with CFAE to patients with all forms of AF, Nademanee et al reported quite high success rates (91% at 1-year follow-up).13
After the report by Nademanee et al, investigators have been evaluating the adjunctive role of CFAE ablation to PVAI. Haissaguerre et al described a stepwise sequential ablation approach for patients with longstanding persistent AF, which included PVAI, linear ablation across the roof of the left atrium between the left and right upper PVs and at the mitral isthmus, ablation at the inferior left atrium toward the coronary sinus and the base of the LAA, and left atrial ablation guided by CFAE mapping.53,54 With this fairly extensive ablation strategy with the procedural endpoint of AF termination, the authors described a consistent prolongation of AF cycle length followed by either organization in ATs (87% of cases) or conversion to sinus rhythm (13% of cases) (Figure 94-8).54 The ATs were subsequently mapped and ablated.
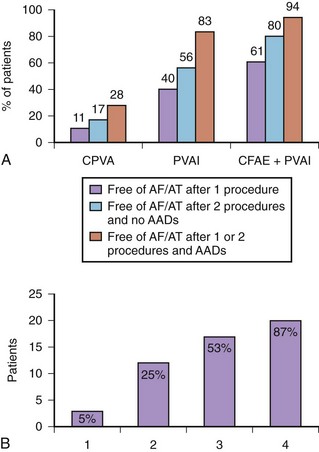
Our group has evaluated the role of CFAE ablation as an adjunct to PVAI in a prospective randomized trial.47 Overall, 144 patients with longstanding persistent AF were randomly assigned to circumferential PV ablation (group 1), PVAI guided by a circular mapping catheter (group 2), or ablation of CFAE followed by PVAI (group 3). After a mean follow-up of 16 months, 11% of group 1 patients achieved sinus rhythm off antiarrhythmic drugs compared with 40% of group 2 and 61% of group 3 patients. Success rates reached 28% in group 1, 83% in group 2, and 94% in group 3 after the second procedure (see Figure 94-8).
In a meta-analysis of six randomized controlled trials on catheter ablation of nonparoxysmal AF that included 360 patients, adjuvant CFAE ablation in addition to PVAI was confirmed to increase the rate of sinus rhythm maintenance (relative risk, 1.35; 95% confidence interval, 1.04 to 1.75; P = .022).48
In our laboratory, procedural termination of AF is not sought as an endpoint.12 In a prospective study on 306 patients with longstanding persistent AF undergoing the first procedure of PVAI plus CFAE ablation, only 6 (2%) patients converted directly to sinus rhythm during ablation, whereas in 172 (56%), the AF organized into AT, which was mapped and ablated, and 128 (42%) patients remained in AF and were cardioverted at the end of the procedure.
Interestingly, after a mean follow-up of 25 ± 6.9 months, 69% of patients remained in sinus rhythm without significant differences between those who had procedural termination or organization of AF and those who remained in AF and received cardioversion. Of note, procedural organization of AF predicted the mode of recurrence, with a significant association with recurrence of AT (P = .022).12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree