Chapter 73 Arrhythmias During Pregnancy
Cardiac arrhythmias during pregnancy, although not a routine problem, are not uncommon. Fortunately, life-threatening tachyarrhythmias in women of childbearing age are infrequent; sustained symptomatic bradyarrhythmias in this population are also exceedingly rare. Additionally, most inherent rhythm disorders manifest long before the patient reaches the childbearing age, suggesting that a more aggressive therapeutic approach may be warranted if the individual plans for a future pregnancy.1 The need to manage arrhythmias in this patient population, however, is likely to become a larger issue as women delay pregnancy until later in life and those with significant congenital heart abnormalities routinely survive into their reproductive years. Few adult cardiologists have had significant exposure to these patients, either in training or in clinical practice. As such, these patients are often approached with a great deal of trepidation, given the recognized pitfall that any management decision on the mother’s behalf could potentially adversely affect the health of the unborn child. This need not be the case if straightforward principles of evaluation and management are followed. Most arrhythmias during pregnancy can be safely and adequately managed with conservative therapies that have excellent outcomes for both mother and child.
Physiological Changes During Pregnancy
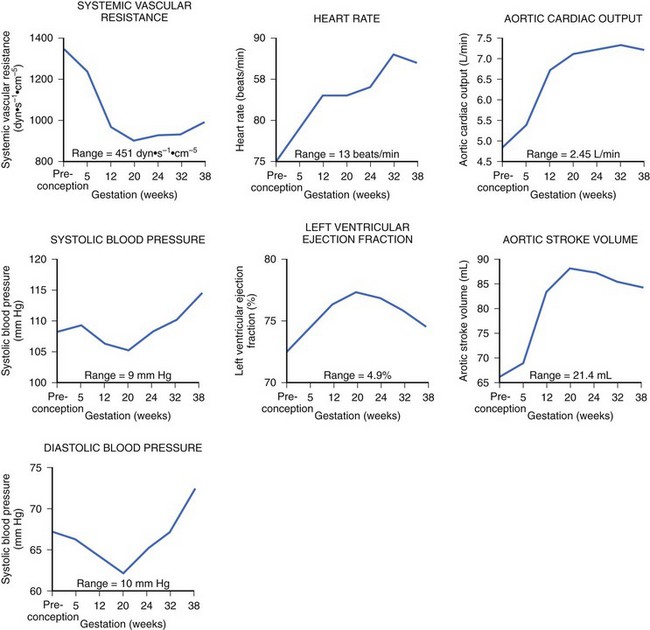
During pregnancy, the mother’s cardiovascular system must accommodate significant hemodynamic changes to meet her own needs and those of the developing fetus. Total body water increases by approximately 5 to 8 liters in a normal pregnancy, and even more so in patients with clinical edema. This process begins during the first trimester and continues until mid-pregnancy, at which time total body water levels stabilize and then gradually diminish until the last week of pregnancy.2 This rise in blood volume results in an approximately 40% increase in cardiac output, significantly increasing the mechanical demands on the maternal heart throughout this period. The increase in cardiac output in early pregnancy is disproportionately greater than the observed increase in heart rate, most likely secondary to the augmentation of stroke volume (Figure 73-1).3 Peripheral vascular resistance is seen to fall throughout pregnancy in proportion to the rise in total body water, resulting in a decrease in blood pressure in early pregnancy; the blood pressure reaches its nadir by mid-pregnancy and returns to baseline levels at term.
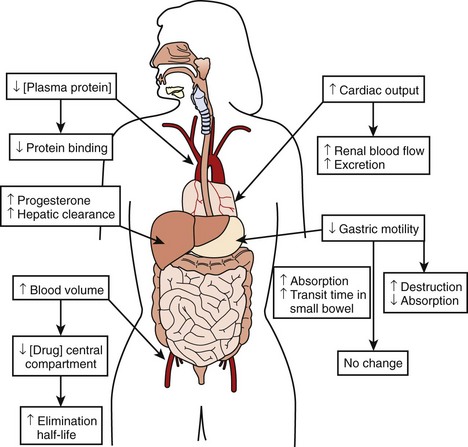
The physiological effects of pregnancy on drug therapy are significant. The observed increase in cardiac output increases renal blood flow by mid-pregnancy by as much as 60% to 80%, resulting in a peak glomerular filtration rate (GFR) that is 50% higher than that before the pregnancy. This GFR level is sustained until the end of pregnancy, resulting in markedly increased metabolism of renally cleared drugs.3 Changes in gastric motility and secretion affect drug absorption both upward and downward.4 Additionally, an increased level of progesterone during pregnancy results in the increased metabolism of hepatically cleared drugs.5 An increased blood volume with the resulting increased volume of distribution may lower the concentration of drugs in the central compartment, increasing their elimination half-life. At the same time, decreases in plasma protein concentration result in less protein binding of susceptible drugs, thus increasing their bioavailability and potential effect.3,6,7 The combined result of these changes makes careful assessment of clinical drug effect essential, as the measured serum drug concentration may be misleading because of altered protein binding. Total measured serum drug concentration may be low because of decreased protein binding, when, in fact, the drug’s active free fraction may have remained unchanged. Thus, although the drug level may appear low, therapeutic efficacy may have been achieved, making increases in drug dosage unnecessary and perhaps dangerous (Figure 73-2).
Principles of Evaluation and Management
There is no place for empirical treatment in pregnant patients.8 This is the cardinal rule that must be followed in the treatment of patients who are pregnant. Careful documentation, correct diagnosis, and correlation of the arrhythmia, whatever it may be, with symptom severity is vitally important. On the one hand, arrhythmias causing hemodynamic compromise to the mother obviously compromise the fetus via reduced placental blood flow and therefore are of primary concern. On the other hand, patients who complain of palpitations, but in whom no arrhythmia can be documented, have a low likelihood of having a life-threatening arrhythmia. In most cases, no further evaluation is warranted.9 In patients with minimal or minor symptoms and a structurally normal heart, the conservative principle “less is better” applies. The need for treatment must be clear. Symptom severity should guide clinical judgment as to whether the benefit of therapy outweighs the risks to both mother and fetus.
History
The recorded history should include detailed questioning of onset, length, and degree of symptoms as well as aggravating and alleviating factors. Symptoms of concern for hemodynamic instability include dizziness, syncope, or near-syncope. Precipitants of arrhythmias such as electrolyte imbalances, renal failure, thyrotoxicosis, and gestational diabetes should be considered.10 Structural heart disease and previously diagnosed tachyarrhythmias are of concern as the women with these conditions are at an increased risk of recurrence or worsening of their arrhythmia throughout the pregnancy. Patients with a history of repaired congenital heart disease will inherently carry the risk of developing scar-related tachyarrhythmias. A new arrhythmia during pregnancy may also be the first manifestation of underlying heart disease.11–13
Physical Examination
From the cardiovascular standpoint, physical examination of the pregnant patient is essentially identical to that of patients who are not pregnant. Normal findings during pregnancy include a widely split first heart sound, expiratory splitting of the second heart sound late in pregnancy, a soft systolic flow murmur believed to represent increased blood flow across the pulmonic valve, and mild peripheral edema.14 Findings of specific interest in a patient presenting with an arrhythmia would include murmurs of mitral stenosis, signs consistent with congestive heart failure, and murmurs consistent with congenital heart disease.
Diagnostic Testing
Electrocardiography
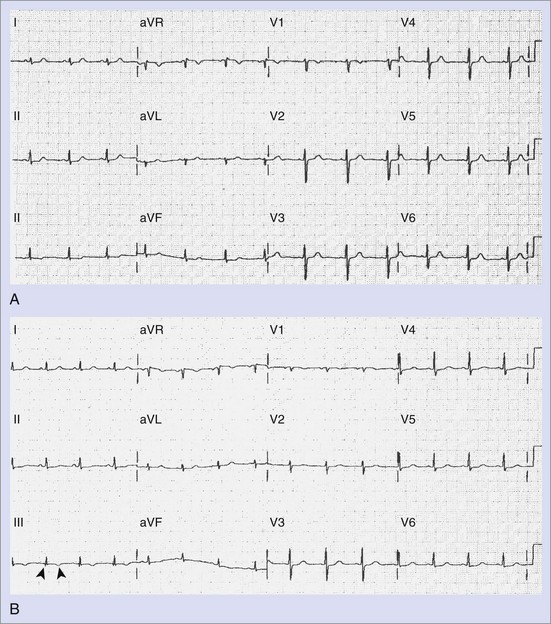
The standard 12-lead electrocardiogram (ECG) is, of course, immensely helpful in making the diagnosis if it can be obtained during an arrhythmia episode. A baseline recording may provide clues to the presence of underlying conduction system disease, chamber hypertrophy, ventricular pre-excitation, QT prolongation, and evidence of arrhythmogenic right ventricular dysplasia. The normal ECG during pregnancy may demonstrate a shift in the QRS axis to the left in the frontal plane with a small Q wave with an inverted T wave in lead III (Figure 73-3, A and B). These are normal changes during pregnancy resulting from a gradual shift in the position of the heart within the thorax.15
Electrophysiology Study
Although currently electrophysiology studies (EPSs) are routinely performed in the diagnosis and treatment of arrhythmias, the use of fluoroscopy for the positioning of catheters during diagnostic and ablative procedures limits this option during pregnancy.8 Radiation exposure of the fetus during the first and second trimesters of pregnancy has been linked to congenital malformations and mental retardation.16 In addition, radiation exposure in utero increases the risk of childhood malignancies, especially leukemia.17–20 If absolutely necessary, an EPS can be performed, with a lead apron draped over the mother’s abdomen; with this approach, good outcomes have been reported in the literature.21 Using intracardiac echocardiography and an electroanatomic mapping system can (and should) also be used for catheter placement and manipulation to minimize fluoroscopic time if ablation appears to be the only effective treatment.22,23 Because of the small, but finite, increased risk of childhood malignancy, routine use of fluoroscopy, even with appropriate shielding, is not recommended.
Cardiac Catheterization
Left heart catheterization performed during pregnancy has been reported infrequently.24 Although rarely indicated for the evaluation of an arrhythmia, knowledge of the coronary anatomy may be crucial to the management of certain patients such as an individual who has experienced aborted sudden cardiac death (SCD) during pregnancy. As the maternal age increases, the occurrence of acute ischemic events during pregnancy may rise. The same precautions mentioned above should be employed, with radiation exposure minimized as much as possible.
Frequency of Arrhythmias Complicating Pregnancy
Life-threatening arrhythmias during pregnancy are rare. Arrhythmias, when present, most commonly present as symptomatic palpitations, which, in most cases, represent an increased level of background atrial or ventricular ectopic activity. Although no single mechanism has been definitively cited, increased mechanical stress, intravascular volume shifts, elevated hormonal levels, and emotional changes occurring during pregnancy likely account for this increase. As most of these women will have structurally normal hearts, this ectopy is generally well tolerated, should be approached as benign, and can be anticipated to substantially resolve in the postpartum period.10,25,26
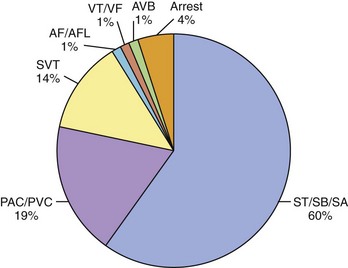
Women with previously diagnosed tachyarrhythmias are at an increased risk of recurrence of their arrhythmia during pregnancy, possibly initiated by increased ectopic activity serving as a trigger for its onset.11,12 Patients with no previous history may present for the first time with an arrhythmia for the same reason. Arrhythmias severe enough to require hospitalization, however, are rare. In a recently published paper, 9 years of hospitalizations at a high-volume obstetric service with the admission diagnosis of “arrhythmia” were reviewed.27 The prevalence of arrhythmia-associated admissions was 166 in 100,000 with an average patient age of 25 years. Sixty percent of arrhythmia admissions comprised a group consisting of patients with sinus tachycardia, sinus bradycardia, and sinus arrhythmia. Premature atrial or ventricular contractions were attributed to 19% of arrhythmia admissions and 14% to supraventricular tachycardia (SVT). Atrial fibrillation (AF) and flutter were counted separately from SVT and comprised only 1% of the arrhythmia admissions. The diagnosis of ventricular tachycardia (VT) or ventricular fibrillation (VF) was made in only 1% of admissions as was atrioventricular (AV) block (Figure 73-4).
Supraventricular Tachycardia
Sinus tachycardia is a normal response to pregnancy. The risk of paroxysmal SVT is generally equally distributed throughout pregnancy, although a rise in the frequency of onset of new or recurrent PSVT in the second and third trimesters has been reported.8,12 Several mechanisms have been postulated to explain the increased incidence, including hemodynamic, autonomic, hormonal, and emotional changes occurring in the pregnant patient. Increased mechanical stress, intravascular volume shifts, elevated estrogen levels, and physiological increases in heart rate have all been implicated.13 AV re-entrant tachycardia—which comprises the pre-excited, Wolff-Parkinson-White (WPW) form of AV reciprocating tachycardia and AV nodal re-entrant tachycardia as well as the non–pre-excited form—is the most common sustained arrhythmia seen in pregnancy and is seen with equal frequency.28,29 Atrial tachycardia, atrial flutter, and AF are encountered most often in patients with structural heart disease, repaired congenital heart disease, and valvular heart disease.
Ventricular Tachycardia
In 1921, Mackenzie reported that nonsustained ventricular arrhythmias were present in approximately 50% of pregnant women in his study.30 The recognized causes of recurrent or paroxysmal VT during pregnancy include arrhythmogenic right ventricular dysplasia, long QT syndrome (LQTS), hypertrophic cardiomyopathy, peripartum cardiomyopathy, and, in rare circumstances, coronary artery disease. Patients without organic heart disease who experience nonsustained VT during pregnancy are at a low risk for subsequent morbidity and mortality.31 Fortunately, very few women during their childbearing years have coronary artery disease, with an even smaller number having re-entrant VT. Multiple cases of sustained VT during pregnancy have been reported. The vast majority of these women have normal hearts and no history of VT before pregnancy. Some of these patients will invariably be found to have catecholamine-sensitive idiopathic VT.32,33 Right ventricular outflow tract (RVOT) tachycardia, for example, is often associated with exercise, typically has a left bundle branch block (LBBB) morphology with an inferior axis of depolarization, and is often responsive to β-blocker therapy (see Chapter 45). Onset of such VT during pregnancy with complete resolution after delivery has been reported in the literature.34 Fascicular VT is also seen in patients with structurally normal hearts. This form of VT generally arises from one of the left ventricular fascicles, most commonly the left posterior fascicle giving a right bundle branch block (RBBB), superiorly directed VT on the 12-lead ECG. This form of VT during pregnancy has also been reported in the literature and is often responsive to calcium channel blockers. Peripartum cardiomyopathy should always be considered with new-onset VT within 6 months of delivery.
Cardiac Arrest
Cardiac arrest during pregnancy is fortunately rare. It is estimated to occur in 1 in 30,000 deliveries as a result of complications occurring during pregnancy, labor, and delivery and in the immediate postpartum period.35 It is important to recognize that in this patient population, the etiology is often different from that for the general population, including amniotic fluid embolism, pulmonary embolism, hemorrhage, and eclampsia.36 Table 73-1 lists the most frequent causes of cardiac arrest in pregnancy. Cardiopulmonary resuscitation (CPR) in the pregnant patient will be reviewed later in this chapter.
Table 73-1 Etiology of Cardiac Arrest in Pregnancy
Arrhythmias in Patients with Previous Tachyarrhythmia and Structural Heart Disease
In women with pre-existing cardiac rhythm disorders, exacerbation of their arrhythmia during pregnancy is common, increasing the risk of fetal complications.37 In a recently published study, Silversides et al reported on the recurrence rates of arrhythmias during pregnancy in women with known cardiac rhythm disorders.12 Initially, in sinus rhythm, 44% developed tachyarrhythmia recurrences during the pregnancy or in the early postpartum period. Nearly 50% of the women studied with a prior history of SVT had recurrence of their SVT complicating the pregnancy. AF or atrial flutter occurred in 52% of patients with a prior history of AF or atrial flutter. Almost all of the patients (96%) known to have AF or atrial flutter before their pregnancy had underlying structural heart disease.12
Congenital heart disease is a significant risk factor for cardiac arrhythmias and is found increasingly in women of childbearing years. Atrial tachyarrhythmias are frequently encountered, as are ventricular arrhythmias that are associated with such congenital conditions as corrected tetralogy of Fallot, hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, and congenital LQTS. Silversides et al reported VT complications in 27% of pregnancies in women with a previous history of VT caused by LQTS or structural heart disease.12
Use of Drugs in the Management of Arrhythmias
Once the correct diagnosis has been made and arrhythmia has been documented, an accounting of the severity of the patient’s symptoms must be undertaken. The reasons for instituting treatment must be clear. Severity of symptoms helps determine whether the risks of therapy outweigh the benefits. The only difference in pregnancy is that the physician must consider the risk/benefit ratio for both the mother and the fetus. An arrhythmia that is hemodynamically compromising to the mother is of major concern because of the resulting compromised blood flow to the placenta. Under normal conditions this will be 6 to 7 L/min during the last trimester. The mother accomplishes this by means of a 15% increase in heart rate and 35% to 40% increase in cardiac output.36 Pharmacologic therapy should be reserved for patients with hemodynamically unstable arrhythmias, whereas bothersome symptoms in a patient with a normal heart should be treated conservatively with reassurance, if feasible.
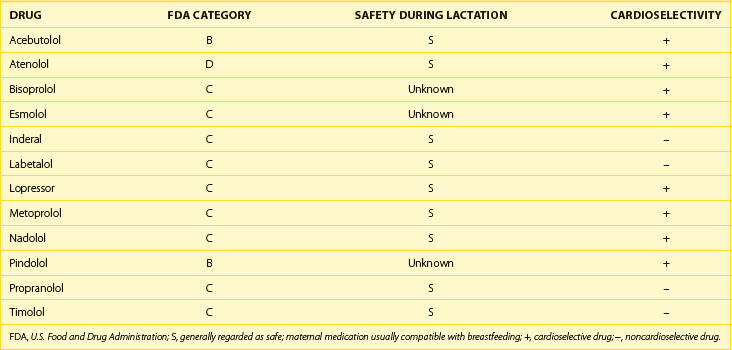
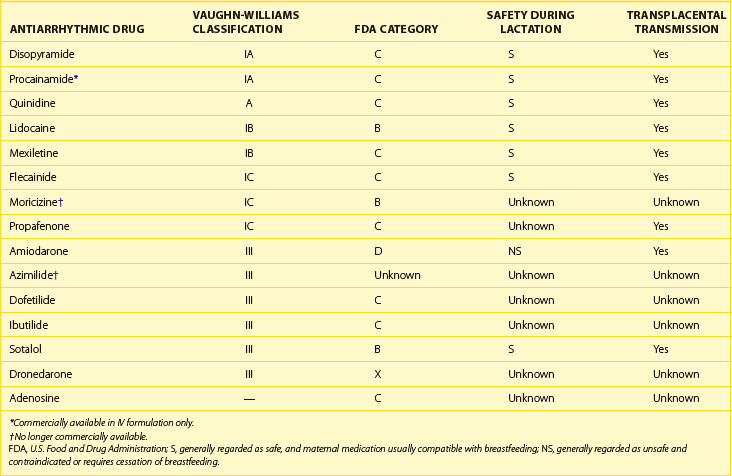
If the decision to initiate drug therapy is made, as few drugs at the lowest effective therapeutic doses as possible must be used. Although this is inherently true for the treatment of all patients, it is particularly important in the pregnant patient. This approach exposes the mother and the fetus to the least possible amount of potential toxins. When feasible, drug choice should be limited to those with a history of safe use in pregnancy. The majority of antiarrhythmic drugs are classified as U.S. Food and Drug Administration (FDA) category C—this means that risk cannot be ruled out—as indicated by animal studies that have suggested risk, but no human studies or controlled studies in either humans or animals that suggest risk exists (Table 73-2).38 Keep in mind that these categories have been criticized as being misleading because they suggest the presence of graded risk as one crosses categories and similar risk among drugs in the same category. This is not accurate, as a wide range of severity of adverse effects exist within classes, and often no distinction can be seen between teratogenic and other toxic effects. For example, if given the choice between a category C drug that is new and one that has a long, safe history of use, the drug with the history of safe use is recommended.25 Of course, a chance of fetal harm is always present when any antiarrhythmic drug is taken during pregnancy, but in the right situation, the potential benefits should outweigh the potential risk. It is unlikely that comprehensive clinical trials will ever be performed in this patient population, for obvious ethical reasons. The publication of clinical case reports involving the use of these drugs in pregnancy is therefore vitally important.
Table 73-2 U.S. Food and Drug Administration Use-in-Pregnancy Ratings
| CATEGORY | INTERPRETATION |
|---|---|
| A | Controlled studies show no risk. Adequate, well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester of pregnancy. |
| B | No evidence of risk in humans. Adequate, well-controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility. |
| C | Risk cannot be ruled out. Adequate, well-controlled human studies are lacking, and animal studies have shown a risk to the fetus or are lacking as well. A chance of fetal harm exists if the drug is administered during pregnancy, but the potential benefits may outweigh the potential risk. |
| D | Positive evidence of risk. Studies in humans or investigational or postmarketing data have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. |
| X | Contraindicated in pregnancy. Studies in animals or humans or investigational or postmarketing reports have demonstrated positive evidence of fetal abnormalities or risk, which clearly outweighs any possible benefit to the patient. |
From the Physicians’ desk reference: PDR, ed 55, Oradell, NJ, 2001, Medical Economics, p 344.
Effects on the Fetus
The physician must consider the stage of pregnancy before deciding on a drug. The risk of teratogenicity is generally higher during the first trimester; after 8 weeks, organogenesis is complete, and the risk to the fetus is substantially reduced. The occurrence of teratogenic abnormalities depends on the drug the fetus is exposed to, the duration of exposure, and genetic predisposition.9 To further complicate management, the absorption, distribution, and excretion of drugs are dramatically altered during pregnancy, as previously described, making monitoring of drug levels difficult and therapeutic effects variable. Drug effects at the time of labor and delivery are also of concern and need to be taken into account.39 In most cases, the method of drug delivery (oral versus intravenous administration) has not been reported to be a significant factor in terms of toxicity. The method of delivery, however, will likely affect the speed and immediate potency of the drug effect and therefore needs to be factored into the decision process.
Lactation and Breastfeeding
Antiarrhythmic drug use during breastfeeding has similar concerns as during pregnancy. Many antiarrhythmic drugs and AV nodal blockers are excreted in breast milk, and thus continued use during breastfeeding must be considered on a case-by-case basis. Both warfarin and heparin are safe for use in nursing mothers who require long-term anticoagulation. Refer to Tables 73-3, 73-4, and 73-5 for current FDA ratings on drug safety during lactation for the most common drugs used to treat arrhythmias.
Table 73-3 Calcium Channel Blocker and Digoxin Therapy During Pregnancy
| DRUG | FDA CATEGORY | SAFETY DURING LACTATION |
|---|---|---|
| Verapamil | C | S |
| Diltiazem | C | S |
| Diltiazem IV | C | NS |
| Digoxin | C | S |
FDA, U.S. Food and Drug Administration; S, generally regarded as safe; maternal medication usually compatible with breastfeeding; IV, intravenous; NS, generally regarded as not safe and contraindicated or requires cessation of breastfeeding.
Atrioventricular Nodal Blockers: Adenosine
Although only limited data are available, it appears that adenosine has no direct effect on the fetus when fetal monitoring is performed during bolus intravenous (IV) administration.40,41 Adenosine, which is a purine nucleoside present in all human cells, characteristically depresses AV nodal conduction and sinus node automaticity. These electrophysiological effects have proven useful for the termination of SVT involving the AV node as part of the re-entrant circuit.42 Because of its rapid onset and short duration of action, it appears to be a safe drug for use during pregnancy, although it remains an FDA category C drug.43
Calcium Channel Blockers
Verapamil is a calcium channel blocker with a long history of use in the management of SVT. It crosses the placental barrier and has been reported to affect fetal cardiovascular activity.44 It is rapidly absorbed but has high first-pass metabolism, with only a small portion excreted unchanged in the urine. Ninety percent of the drug is plasma protein bound. Congenital defects in association with verapamil have not been reported. Verapamil is an FDA category C drug.
Diltiazem is a relatively newer drug than verapamil, so less information on the clinical history of its use in pregnancy is available. Nevertheless, it has been used in the treatment of premature labor without reported congenital abnormalities and thus should be safe for use in arrhythmias.45 Diltiazem is an FDA category C drug (see Table 73-3).
Digoxin
Digoxin is a cardiac glycoside, which has a long history of use in pregnancy for the management of supraventricular arrhythmias, although it remains classified as an FDA category C drug. Elimination of the drug is predominantly renal. Digoxin crosses the placenta readily, with fetal plasma concentrations similar to maternal values within 30 minutes.46 These agents have also been administered maternally to manage fetal tachyarrhythmias.47,48 Digoxin is not teratogenic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree