Historical perspective
The original concept of cardiac transplantation dates to the 1890s, with the work of Alexis Carrel and subsequently Mann and colleagues at the Mayo Clinic in the 1930s.1 The modern era of human cardiac transplantation started with pioneering work by Vladimir Demikhov in Russia in the 1940s2 and Norman E Shumway’ s group at Stanford University throughout the 1950s, the latter associated with the development of cardiopulmonary bypass3 and perfection of the surgical technique. By the mid 1960s, it was concluded by Shumway that the major barrier to successful mammalian cardiac allotransplantation was rejection.
The first human cardiac transplant was performed by Dr Christiaan Barnard at Cape Town, South Africa, in 1967. Local irradiation, azathioprine, prednisone and actinomycin C were used as immunosuppression. The patient survived 18 days but ultimately succumbed to Pseudomonas pneumonia.4
Worldwide, further attempts at human cardiac transplantation met with poor outcomes. Most groups discontinued their efforts, but the Stanford group implemented criteria and protocols for detection of acute rejection early post transplant. Initially, this involved ECG, echocardiographic and clinical parameters, but in 1973 histologic criteria on endomyocardial biopsy were developed and remain the mainstay of rejection surveillance. The discovery of ciclosporin A by Professor Jean-Fran ç ois Borel in 1976 and its subsequent introduction into clinical practice after successful animal studies5 was the breakthrough in the prevention of rejection which allowed successful cardiac transplantation. Despite these advances, the number of transplants performed worldwide has plateaued, primarily due to lack of donor organ supply.6 This has instigated research and development of new therapeutic avenues such as mechanical device therapy.
The International Society for Heart and Lung Transplantation (ISHLT) has reported outcome data on transplant recipients on an annual basis for the past 24 years. The most recent data are summarized below.7
Demographics
The primary indication for cardiac transplantation has remained unchanged in recent years, almost evenly divided between ischemic (41%) and non-ischemic cardiomyopathy (45%). The remaining indications represent a minority of patients with valvular and congenital heart disease and those retransplanted (all 3%). An increasing number of patients are now on inotropic (40%) or mechanical support (including left ventricular assist devices [LVADs], 27%) at the time of cardiac transplantation.
The age of both donors and recipients has increased in the past 20 years. Almost 25% of cardiac transplant recipients in the past year were over the age of 60 years, with a relative fall in numbers of recipients aged 40–49 years. Donors over the age of 50 years now comprise >12% of donors worldwide. Twenty percent of European donors are over 50 years old, whereas in the US that figure is closer to 10%.
Outcomes
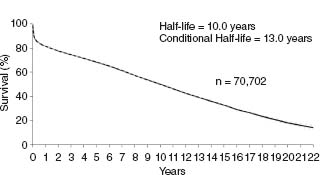
Since the ISHLT started reporting outcomes in 1982, early survival (up to one year) post transplantation has improved steadily.8 However, long-term mortality has not changed and the current transplant half-life is 10 years worldwide for adult and pediatric cardiac transplant recipients combined, representing a steady improvement over the past 20 plus years due to the improvements in early survival (Fig. 68.1). In particular, survival for patients retransplanted has improved and is currently similar to those transplanted for the first time (approximately 85% one-year survival in those surviving the first year).
The risk factors for death within one year and, in those surviving the first year, for death within five years are shown in Table 68.1. In the current era, the most significant risk factors for death post cardiac transplantation in the first year remain requirement for short-term extracorporeal mechanical support post transplantation, congenital heart disease as an indication for transplantation and presence of insulin-requiring diabetes mellitus pretransplant. After the first year, the highest risk factors for death within the first five years are need for retransplantation, the development of cardiac allograft vasculo pathy and insulin-requiring diabetes mellitus prior to transplantation.
Table 68.1 Risk factors for mortality in first year post cardiac transplantation
| 95% confidence interval | |
Risk factors for death within 1 year* | |
Need for short-term extracorporeal mechanical support | 2.11, 5.42 |
Adult congenital heart disease | 1.47, 3.10 |
Insulin-requiring diabetes mellitus | 1.02, 3.41 |
Mechanical ventilation at time of transplant | 1.36, 2.46 |
Prior pregnancy | 1.05, 1.57 |
Previous history of CVA | 1.00, 1.67 |
Recent infection (within 2 weeks) requiring IV antibiotics | 1.04, 1.55 |
LVAD in situ ‡ | 1.05, 1.55 |
Prior sternotomy | 1.05, 1.42 |
Risk factors for death within 5 years § | |
Retransplantation | 1.55, 9.57 |
Early-onset coronary artery vasculopathy (within first year) | 1.55, 2.70 |
Diabetes mellitus pretransplant | 1.25, 1.86 |
Treated infection during initial transplant hospitalization | 1.12, 1.66 |
Treated rejection episode in first year | 1.06, 1.50 |
Ischemic vs non-ischemic cardiomyopathy pretransplant | 0.99, 1.45 |
Reproduced with permission from reference6, courtesyof the ISHLT, 2007.
* Data based on transplants performed January 2002 through June 2005, n = 7024.
‡ Data based on a predominance of pulsatile devices having been implanted at the time of audit.
§ Data based on transplants performed January 1999 through June 2001, n = 4079.
The commonest causes of death up to 30 days, in the first year and up to five years, are shown in Table 68.2. Graft failure remains the commonest cause of death within 30 days, and includes ischemic/reperfusion injury, right heart failure and acute rejection. Beyond 30 days, infection becomes prominent as the commonest cause of death up to the first year (33% of deaths). After this timepoint, cardiac allograft vasculopathy is the most frequent cause of death, representing 30% of all deaths within five years post cardiac transplantation, closely followed by malignancy (22% of deaths).
Table 68.2 Leading causes of death post cardiac transplantation
| Proportion of all deaths | |
Up to 30 days ( ∼ 10% mortality) | |
Graft failure | 40% |
Multiorgan failure | 14% |
Infection * | 13% |
Up to 1 year ( ∼ 15% mortality) | |
Infection * | 33% |
Graft failure | 18% |
Acute rejection | 12% |
5 years ( ∼ 30% mortality) | |
Cardiac allograft vasculopathy | 30% |
Malignancy | 22% |
Infection * | 10% |
* Excluding CMV infection
The current AHA/ACC recommendations on selection of adult patients for cardiac transplantation are set out in their practice guidelines for the management of chronic heart failure in the adult.9 The primary focus is on patients with severe functional impairment and/or dependence on inotropic support. Rarely, recurrent malignant arrhythmias refractory to medical therapy and debilitating refractory angina pectoris secondary to severe non-revascularizable ischemic heart disease may be indications. Contraindications to cardiac transplantation are all relative and dependent on how modifiable they are before surgery. They include pulmonary disease, pulmonary hypertension, diabetes with complications, systemic disease (including malignancy) and peripheral vascular disease. Age is also included, but this is an area of controversy as data regarding outcomes in older recipients have been conflicting.10,11 The indications for and contraindications to cardiac transplantation are summarized in Box 68.1.
Selection of a donor requires a number of criteria to be met. First, national (and/or regional criteria where these vary, as in the US) for brain death must be met. The ECG and the echocardiogram should be normal. If a donor older than 45 years is being considered, coronary angiography is performed to exclude significant coronary artery disease. Otherwise, the risk factor profile for coronary artery disease should be low and there should be no evidence of untreated acute infection or systemic malignancy. The HIV and hepatitis screens should be checked and confirmed negative. Potential donors with cardiac trauma are usually excluded.
The matching of a suitable donor to a recipient is dependent on a limited number of key issues.
Blood type
ABO matching is mandatory. Matching of rhesus status is not required.
Body size
Generally, the donor should be at least 80% of the body weight of the recipient.
Pulmonary vascular resistance
Where this is high (generally in excess of 4–5 Wood units) in the recipient, a larger donor heart is usually selected to ensure adequate right ventricular functional reserve. In addition to pulmonary vascular resistance, the pulmonary artery pressure is also considered, and in particular the assessment of reversibility of high pulmonary pressures seen in some patients with chronic heart failure.
BOX 68.1 Cardiac transplantation – indications and contraindications
Absolute indications
- Hemodynamic compromise secondary to heart failure
- Refractory cardiogenic shock
- Dependence on IV inotropic support for adequate organ perfusion
- Peak VO2 <10mL/kg/min
- Severely limiting non-revascularizable ischemic heart disease affecting daily living
- Recurrent symptomatic VT refractory to therapy
Relative indications
- Peak VO2 11–14mL/kg/min with significant limitation of functional capacity
- Recurrent unstable angina refractory to current therapy
- Recurrently labile fluid balance/renal function in chronic heart failure despite full patient compliance with therapy
Insufficient indications
- Presence of the following without other indications for transplantation:
- Impaired LV systolic function
- Previous history of class III–IV heart failure
- Peak VO2 >15mL/kg/min
Contraindications (all relative)
- Pulmonary hypertension (must have evidence of significant reversibility at right heart catheterization)
- Age (controversial – see text)
- Parenchymal lung disease with significant pulmonary function abnormalities (<50% predicted)
- Co-existent systemic illness (e.g. malignancy, infiltrative disorders, infection)
- Acute pulmonary embolus
- Severe peripheral vascular disease
- Irreversible renal and hepatic dysfunction
- Diabetes with severe end-organ damage
- Severe obesity
- Severe osteoporosis
- Psychosocial issues
- Drug addiction, including nicotine
Recipient stability
Where the recipient is unstable (status 1 vs status 2), the urgency of finding a suitable donor heart occasionally requires some compromise on an “ideal” match as outlined above.
Geographic location of donor
This always needs to be considered to ensure the lowest possible cold-ischemic time of the heart after it has been explanted from the donor. Once this rises beyond four hours, outcomes may be compromised. This is accentuated if there is significant hypertrophy of the donor organ.
Anti-HLA a ntibody t iter
Due to the short time window of permitted cold-ischemic time in the setting of heart transplantation, unlike renal transplants, HLA cross-matching is only performed if titer of preformed antibodies in the recipient (PRA level) is significant. The PRA level is part of the routine pretransplant assessment of the recipient. This titer of recipient anti-HLA antibodies reflects the degree of sensitization of the patient to foreign antigens of HLA A, B and DR subtype. This is performed by incubating recipient serum in different wells with a random panel of donor lymphocytes. The result is represented as a percentage of total wells on a panel which show evidence of a positive reaction, hence the term PRA.12 Numerous variations in methodology exist, and most recently have included flow cytometric virtual cross-match.13 However, there is also variation in the interpretation of results–most programs consider a titer greater than 10% to be significant. Some institutions have considered any elevation or only titers greater than 20–25% to be of significance.14
The importance of the pretransplant PRA level has been known for some time and elevated PRA titers have been associated with increased risk of hyperacute rejection, antibody and cell-mediated rejection, and also cardiac allograft vasculopathy.15,16 Therefore, patients with significantly elevated pretransplant PRA levels (> 10% according to the American Society of Histocompatibility and Immunogenetics and UNOS) require HLA cross-matching to a donor organ.17
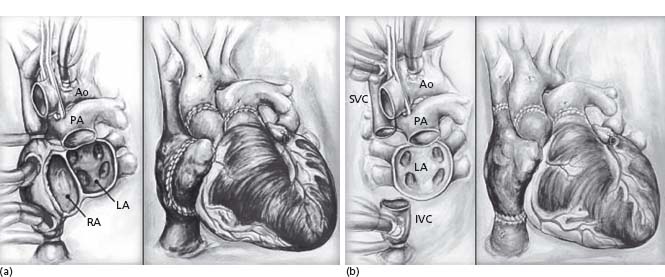
The original operative technique described by Shumway involved removal of the native heart and anastomosis of the recipient heart at mid-atrial level bilaterally (Fig. 68.2).18 This so-called “biatrial anastomosis” technique was shown to increase the incidence of atrial arrhythmia, right atrial thrombus and tricuspid valve dysfunction.19,20 The “bicaval technique” involves preservation of the recipient pulmonary veins and anastomosis of a small cuff of recipient left atrial tissue to the donor left atrium, but retaining the anastomosis of the venae cavae and thus sparing integrity of the donor right atrium.21 Recent evidence has shown short-term clinical benefits of the bicaval technique when compared to the biatrial technique, with enhanced preservation of atrial contractility, sinus node function and tricuspid valve competence, but there is insufficient evidence to date on long-term outcomes.22
Immunosuppression
There are initial induction strategies and long-term chronic immunosuppression regimens. Induction therapy use has increased from 42% in 1995 to 52% in 2005, with use of antithymocyte globulin stable at 20–22% but the more traditional anti-T cell receptor monoclonal antibody OKT3 used in less then 3% of cardiac transplants in 2005.6 The rationale for induction therapy is to reduce the risk of early acute rejection through enhanced immunosuppression and, in addition, the risk of postoperative renal dysfunction through a delay in commencement of calcineurin inhibitor therapy.23 However, induction therapy is not without risk and substantive evidence has linked it to an elevated risk of development of post-transplant lymphoproliferative disorder.24 Therefore, the use of induction therapy varies, with many centers avoiding its use completely. The newer interleukin-2 antagonists are currently under evaluation.26
Maintenance immunosuppression varies among centers. The traditional model of a calcineurin inhibitor (ciclosporin or tacrolimus), an antiproliferative agent (azathioprine or mycophenolate) and a steroid is usual, but use of tacrolimus in preference to ciclosporin has increased in recent years.6 Two randomized clinical trials have studied tacrolimus in direct comparison with ciclosporin. A European-based multicenter study on over 300 patients showed a significantly lower incidence of severe rejection in the tacrolimus-treated patients at six months but no difference in patient or graft survival at 18 months.27 A smaller study from the US in which patients did not receive induction therapy showed no difference in survival or incidence of severe rejection between groups but a significantly lower incidence of renal dysfunction and hyper-triglyceridemia in the tacrolimus-treated patients.28 In addition, a non-significant trend was observed towards a lower requirement for antihypertensive therapy in the tacrolimus group.
Mycophenolate is currently used in over 70% of transplanted patients at one year. The advantages of mycophenolate over azathioprine post cardiac transplantation include a reduced incidence of acute rejection and mortality, and possibly even of the incidence of cardiac allograft vasculopathy.29 The underlying mechanisms for this benefitmay include preferential anti-B cell activity compared to azathioprine, and reduced production of anti-HLA antibodies post transplantation.30,31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree