History and Overview of Cardiac Transplantation
Despite major advances in the treatment of severe myocardial failure, a sizable number of patients with terminal or progressive myocardial dysfunction are fated to die or be severely limited by symptoms. In these patients, biological replacement of the heart (cardiac transplantation) has become standard therapy and is widely accepted as a modality for prolonging life and improving its quality in carefully selected patients. As technologic and engineering advances occur, support or replacement of the heart by mechanical devices (mechanical circulatory support) is additionally providing alternative or complementary treatment modalities for many of these patients.
Interest in developing surgical techniques to interpose a functioning heart into a recipient’s circulation dates back at least to the early part of the twentieth century. In 1905, Carrel and Guthrie1 described the heterotopic transplantation of a functioning donor heart into the neck of a dog. The heart in that model functioned together with the recipient’s heart in the circulation but was not capable of supporting the circulation. Although the exact anatomic connections were not described in detail, this apparently nonworking model of heterotopic transplantation beat regularly for approximately 2 hours before the blood clotted in all of the chambers. Carrel Guthrie2 developed innovative surgical techniques for vascular anastomosis at the University of Chicago, and those advances set the stage for anastomosis, leading to organ transplantation. This work was partially responsible for Carrel’s being awarded the Nobel Prize for medicine and physiology in 1912.
It was not until 1933 that Mann and coworkers3 at the Mayo Clinic published their seminal report of a technique for heterotopic heart transplantation with circulatory loading of the RV. Because this was a working model, the chambers did not clot immediately, and the hearts in their dogs beat for a mean of 4 days. Mann and coworkers perceived several important surgical points, including the importance of avoiding ventricular distention and air embolism and the prevention of thrombosis by heparin. Their most incisive observation was that failure of a transplanted heart was not always caused by faulty surgical technique “but to some biologic factor which is probably identical to that which prevents survival of other homotransplanted tissues and organs.“ In what was undoubtedly the first description of acute allograft rejection, Mann and coworkers recount: “When the heart was removed just before it became quiescent . . the surface of the heart was covered with mottled areas of ecchymoses . . histologically the heart was completely infiltrated by large mononuclears and polymorphonuclears.“3 It took another 30 years to understand and manipulate the “biologic factor“ these authors had described as limiting the survival of allograft organs. In 1960, Lower and Shumway4 performed orthotopic heart transplants in dogs using cardiopulmonary bypass and topical hypothermia for donor heart preservation. The dogs survived between 6 and 21 days and died of rejection. Lower and Shumway also recognized that “if the immunologic mechanisms of the host were prevented from destroying the graft, in all likelihood it would continue to function adequately for the normal lifespan of the animal.“ Their technique, involving anastomoses at the midatrial level and at the supravalvular level in the great vessels, remained the basis of cardiac transplant technique through the 1990s.
In the early 1960s, the concept of pharmacologic immunosuppression was introduced; it ushered in the marriage of surgical and medical knowledge that is known today as the field of organ transplantation. Immunosuppression was, of course, seen as a means to mitigate the “biologic factor“ that otherwise limited organ graft survival. The first clinical transplants were of the kidney, a logical choice because hemodialysis was then available as a backup treatment if the graft failed, and the field has flourished since the early 1960s.5
The first human heart transplant was performed in South Africa in 1967,6 followed shortly thereafter by the first US heart transplant by Shumway and colleagues at Stanford in 1968. A flurry of transplant activity began at many centers, but the initial enthusiasm subsided as it became evident that postoperative survival was limited by a variety of complex medical problems, including opportunistic infections and graft rejection. Most major centers discontinued their heart transplant programs in the early 1970s, and it was not until the introduction of cyclosporine-based immunosuppression in 1980 and the demonstration of the attendant improvement in survival rates7 that the procedure reemerged as a widely accepted therapy for end-stage heart disease. By the 1990s, many tertiary care and academic centers had established programs for heart transplantation, and most medical care payers in the United States, including the federal government, provided coverage for such care. Currently, the number of heart transplants performed worldwide is estimated to be more than 5000 procedures annually, a level that is currently limited by donor availability.8
Recipient Selection and Management
Cardiac transplantation remains the treatment of choice for patients with end-stage cardiac disease with severe functional limitation, usually New York Heart Association (NYHA) functional class III or IV, whose symptoms are refractory to management with medications; electrophysiologic device therapy such as cardiac resynchronization; and, in some cases, surgical intervention. Table 29–1 lists the currently accepted indications for transplantation. The primary indications for adult heart transplantation today continue to be primarily divided between nonischemic cardiomyopathy (44%) and coronary artery disease (35%) (Fig. 29–1).8
| Systolic heart failure with severe functional limitations or refractory symptoms despite maximal medical and device therapy |
| LVEF usually <35%, but a low LVEF is not an adequate indication for transplantation |
| NYHA functional class III-IV |
| Maximal oxygen uptake (VO2max) of ≤12-14 cc/kg/min exercise testing |
| Cardiogenic shock not expected to recover |
| Acute myocardial infarction |
| Acute myocarditis |
| Ischemic heart disease with intractable angina not amenable to surgical or percutaneous revascularization and refractory to maximal medical therapy |
| Intractable ventricular arrhythmias, uncontrolled with standard antiarrhythmic therapy, device therapy, or ablative therapy |
| Severe symptomatic hypertrophic or restrictive cardiomyopathy |
| Congenital heart disease in which severe, fixed pulmonary hypertension is not a complication |
| Cardiac tumors with a low likelihood of metastasis |
Although the indications for cardiac transplantation are generally well accepted, identifying the subgroup of patients that is most likely to derive a benefit from transplantation can be challenging. Advances in medical and device therapy for heart failure over the past decade, including the judicious use of β-blockers in patients with severe heart failure, the introduction of aldosterone antagonists, and the increasing use of implantable defibrillators and cardiac resynchronization therapy, have resulted in significant improvements in the 1-year survival rates of patients with advanced heart failure (NYHA functional class III-IV).9-12 Patients treated with aggressive medical and device therapy have 1-year survival rates approaching 90%, comparable to 1-year survival rates after transplantation.8,12 Additionally, a small percentage of patients exhibit improvements in their left ventricular (LV) systolic function and exercise tolerance after mitral valve surgery or high-risk coronary bypass surgery aimed at restoring blood flow to areas of hibernating myocardium. Electrophysiologic mapping and ablative therapy can provide control of incessant ventricular arrhythmias in a subset of patients who have failed or cannot tolerate antiarrhythmic therapy. Finally, cessation of excessive alcohol intake or slowing of the ventricular rate with drugs or atrioventricular (AV) nodal ablation in patients with rapid heart rates can occasionally result in a dramatic reversal of the heart failure.13 Thus, many transplant centers have found that as many as 30% to 50% of patients referred for heart transplants can be stabilized by an aggressive, multidisciplinary approach, such that transplantation can be avoided or delayed. As a result, most heart transplant centers have evolved into centers for advanced heart failure management in addition to providing the opportunity for transplantation.
In patients undergoing transplant evaluation, measurement of peak oxygen consumption (VO2) during cardiopulmonary exercise testing provides an objective assessment of functional capacity and is more useful than NYHA classification, ejection fraction, or other markers of heart failure severity for assessing prognosis and determining the optimal timing of listing for transplantation. Patients with a peak VO2 of more than 14 mL/kg/min have 1- and 2-year survival rates that are comparable or better than those achieved with transplantation, and these patients should be managed medically and undergo serial exercise testing.14 Patients with a peak VO2 between 10 and 14 mL/kg/min constitute an intermediate-risk group in which continued medical therapy may offer a survival benefit similar to heart transplantation among selected patients that are able to tolerate β-blockers, have low-risk Heart Failure Society Scores (HFSS), and have the protection of an internal defibrillator.15,16 The HFSS is a predictive model calculated from seven prognostic variables that are commonly obtained during the transplant evaluation process (Table 29–2).16 In patients tolerating β-blockers, a peak VO2 of less than 12 mL/kg/min has been suggested as an appropriate threshold to identify individuals who are likely to derive a survival benefit from transplantation.17 Patients with a peak VO2 of 10 mL/kg/min or less, regardless of β-blocker use, have significantly reduced survival rates with medical therapy compared with cardiac transplantation, and these patients should be listed for transplantation.14,18
| Variable | Coefficient |
|---|---|
| Ischemic cardiomyopathy (1 = yes; 0 = no) | +0.6931 |
| Resting heart rate (beats/min) | +0.0216 |
| LVEF (%) | −0.0464 |
| Mean BP (mm Hg) | −0.0255 |
| IVCD (QRS ≥120 ms) (1 = yes; 0 = no) | +0.6083 |
| Peak VO2 (cc/kg/min) | −0.0546 |
| Serum sodium (mEq/L) | −0.0470 |
Appropriate candidates for cardiac transplantation should have severe functional limitations and limited life expectancy from their heart disease and should be free of established contraindications (Table 29–3). Although many of the exclusion criteria are relative contraindications, the degree to which they are interpreted and applied may vary considerably among transplant programs.
| Irreversible severe pulmonary arterial hypertension: |
| PVR >5 Wood units |
| PVRI >6 |
| Transpulmonary gradient >16-20 mm Hg |
| PA systolic pressure >50-60 mm Hg or >50% of systemic pressures |
| Advanced age (>70 y) |
| Active systemic infection |
| Active malignancy or recent malignancy with high risk of recurrence or progression |
| Diabetes mellitus with: |
| End-organ damage (neuropathy, nephropathy, proliferative retinopathy) |
| Poor glycemic control (HbA1c >7.5) |
| Marked obesity (BMI >30 kg/m2 or >140% of IBW) |
| Severe peripheral arterial disease not amenable to revascularization |
| Irreversible severe renal, hepatic, or pulmonary disease (unless combined organ transplantation is considered) |
| Recent or unresolved pulmonary infarction |
| Psychosocial factors that may impact on the patient’s ability to comply with a complex medical regimen: |
| History of poor medical compliance |
| Lack of adequate support system |
| Uncontrolled psychiatric illness (anxiety, depression, psychosis) |
| Active or recent substance abuse (alcohol, tobacco, or illicit drugs) |
The upper age limit for cardiac transplant recipients has expanded considerably over the past decade, with many centers advancing their official chronological age limits to 65 years and considering highly selected patients older than age 65 years on a case-by-case basis. It is now recognized that physiologic age may be a more important than chronologic age with respect to survival and rehabilitation potential. As a result, many programs are moving away from fixed upper age limits and instead focus on a patient’s functional status; integrity of major organ systems; and the presence of comorbidities that might impact survival, rehabilitation potential, and quality of life.
Pulmonary hypertension from chronic elevations of LV end-diastolic pressure and neurohormonal activation is a common complication of long-standing heart failure and can result in irreversible changes to the pulmonary vasculature over time if left unrecognized and untreated. Early in the years of clinical experience with heart transplantation, it was discovered that a normal donor right ventricle (RV) is unable to increase its external workload acutely to overcome elevated pulmonary vascular resistance (PVR), resulting in acute RV failure and cardiogenic shock postoperatively. Elevated PVR remains a strong risk factor for RV failure and early postoperative mortality in the modern era. Potential heart transplant candidates must therefore undergo measurements of pulmonary artery pressures and calculation of PVR in the cardiac catheterization laboratory or in the intensive care unit as part of their transplant evaluation. A pulmonary artery systolic pressure above 50 to 60 mm Hg, a PVR value above 5 Wood units (∼ 400 dynes·s/cm5), a PVR index above 6, or a transpulmonary gradient above 15 to 20 mm Hg is usually considered prohibitive of successful heart transplantation unless the pulmonary artery pressures and PVR can be reduced to acceptable levels while maintaining a systolic blood pressure above 85 mm Hg.19 Pharmacologic interventions for reducing PVR include administration of intravenous (IV) agents such as nitroprusside, prostaglandin E1, milrinone, and dobutamine or use of inhaled nitric oxide. Occasionally, long-term offloading of the LV using an intraaortic balloon pump or LV assist device (LVAD) has been used as a bridge to transplantation candidacy in patients with severe, refractory pulmonary hypertension.
Patients must be free of active infection and malignancy before transplantation because both processes can be exacerbated by the immunosuppression that is required after transplantation to prevent rejection. The presence of an active systemic infection or severe localized infection is often considered a temporary contraindication to transplantation. Patients with a history of infection should not be activated or reactivated on the transplant waiting list until there is sufficient evidence that the infection is resolved or under control, as demonstrated by absence of fever for a minimum of 72 hours on appropriate antibiotics, a normal white blood cell count, negative blood culture results, and resolving signs or symptoms of infection.
The evaluation of patients with chronic viral infections such as hepatitis B, hepatitis C, or HIV remains controversial. Although most transplant centers accept candidates who are hepatitis C antibody positive, asymptomatic patients with evidence of hepatitis B surface antigenemia have a high risk of developing subsequent clinical liver disease with the institution of immunosuppression and are considered poor transplant candidates by many centers.20,21 Although HIV infection was once considered an absolute contraindication to transplantation because of concerns that immunosuppressive therapy may hasten the progression of HIV disease and increase the risk of opportunistic infections or malignancy, these concerns have not been validated to date. It is now believed that the presence of HIV infection in itself should not serve as a contraindication for heart transplantation but that HIV-infected patients should be carefully evaluated for extent of their disease and the presence of opportunistic infections.22-24 Although some heart transplant centers have now successfully transplanted a few patients with HIV infection, the overall experience with this group remains limited.23
In general, patients with active malignancies, with the exception of nonmelanoma cutaneous cancers, primary cardiac tumors restricted to the heart, and low-grade neoplasms of the prostate, should be excluded from cardiac transplantation. However, preexisting neoplasms are diverse with respect to their response to therapy and risk of recurrence. Consultation with an oncologist should be obtained for any patient with a history of previous or active malignancy to assess the risk of tumor recurrence. Cardiac transplantation should be considered when the risk of tumor recurrence is low based on the tumor type, response to therapy, and negative metastatic workup. The specific amount of time to wait before transplantation after neoplasm remission should depend on the aforementioned factors, and no arbitrary time period for observation should be used.
Potential cardiac transplant recipients are screened for the existence of other conditions or systemic diseases that may independently limit their survival or rehabilitation potential. Obesity has been associated with an increased risk of infection, wound healing complications, metabolic derangements, acute rejection, cardiac allograft vasculopathy, and mortality after heart transplantation.24-26 Furthermore, the need for corticosteroids and the likelihood of further weight gain after transplantation compound these problems.27 In severely obese patients, weight loss is recommended to achieve a body mass index below 30 kg/m2 or percent ideal body weight of less than 140% before they are listed for cardiac transplantation.19
The presence of preexisting insulin-requiring diabetes mellitus was once considered a relative contraindication to heart transplantation because of concerns regarding diminished survival, increased infection rates, and worsening glycemic control with the initiation of corticosteroid immunosuppression. In recent years, several reports have demonstrated similar short- and long-term survival rates in diabetic and nondiabetic groups, as well as similar rates of infection, rejection, renal function, and cardiac allograft vasculopathy.28-30 Although the safety and efficacy of heart transplantation in these very carefully selected patients has been documented in the literature, most transplant programs continue to consider the presence of diabetes with end-organ damage (proliferative retinopathy, neuropathy, or nephropathy) a relative contraindication to transplantation. Because corticosteroid therapy may worsen glycemic control in patients with preexisting diabetes, the presence of poorly controlled diabetes, as manifested by a hemoglobin A1c value above 7.5 mg/dL [DS1]despite aggressive diabetic education and management, is also considered a relative contraindication for transplantation.
Other comorbid conditions must be considered on an individual basis, but irreversible organ dysfunction such as pulmonary fibrosis, severe emphysema, and hepatic or renal dysfunction out of proportion to that predicted as a consequence of severe heart failure are strong relative contraindications. Selected patients with irreversible renal or hepatic dysfunction may be considered for multiorgan transplantation.31-34
Advanced noncardiac vascular disease, in the form of symptomatic cerebrovascular disease or peripheral vascular disease that is not amenable to revascularization, is considered a relative contraindication to transplantation if the condition is expected to limit survival or impair rehabilitation after transplantation.
Experience has shown that pulmonary infarcts have a high probability of becoming pulmonary abscesses after the institution of immunosuppression. For this reason, potential recipients who sustain a pulmonary infarction are usually temporarily removed from the waiting list until the infarct resolves as shown radiographically.
All cardiac transplant candidates should undergo a careful psychosocial assessment with emphasis on current and previous substance abuse history, compliance with medical therapy and follow-up, comprehension of and ability to follow a complex medical regimen, and adequacy of social support. Patients should also be screened for psychiatric conditions that may affect the aforementioned factors. Active substance abuse, including tobacco, excessive alcohol, and illicit drug use, is widely accepted as a contraindication for heart transplantation. Many transplant centers require documented abstinence from substance abuse for a period of 6 to 12 months prior to transplantation. In addition, patients may be required to complete a structured rehabilitation program before being eligible for transplantation.
Donor Selection and Management
Acceptance of the concept of irreversible brain death, both legally and medically, has been integral to the emergence of organ transplantation in the modern era. The most widely accepted set of guidelines for the determination of brain death was set out in the President’s Commission Report in 1980 and are summarized in Table 29–4.35 The most common causes of brain death include intracranial hemorrhage, blunt traumatic injury to the head, penetrating traumatic injury, and anoxic brain injury. Patients with irreversible brain injury accompanied by the intent to withdraw life support are also considered potential organ donors.
| Clinical evaluation |
|---|
| Mechanism of brain injury is sufficient to account for irreversible loss of brain function |
| Absence of reversible causes of CNS depression |
| CNS depressant drugs |
| Hypothermia (< 32°C) |
| Hypotension (MAP <55 mm Hg) |
| Absence of NMBs that may confound the results of the neurologic examination |
| No spontaneous movements, motor responses, or posturing |
| No gag or cough reflexes |
| No corneal or pupillary light reflexes |
| No oculovestibular reflex (cold calorics) |
| Confirmatory tests |
| Apnea test for minimum of 5 min showing |
| No respiratory movements |
| pCO2 >55 mm Hg |
| pH <7.40 |
| No intracranial blood flow |
After a potential donor is identified, the procurement process is initiated by contacting the local, or “host,“ organ procurement organization (OPO). The host OPO is responsible for obtaining consent for organ donation, verifying pronouncement of brain death, evaluating and managing the donor, and equitably allocating the donor organs. The process of donor evaluation begins with a detailed history and physical examination, focusing on cause of death, past medical history, donor height and weight, and clinical course. Basic laboratory studies, including a complete blood count, metabolic panel, ABO blood typing, and viral serologies (hepatitis B and C, HIV, human T-cell leukemia virus, Ebstein-Barr virus [EBV], and cytomegalovirus [CMV]) are ordered. Additional studies include chest radiography, 12-lead electrocardiography (ECG), and echocardiography. To be considered suitable donors for cardiac transplantation, brain-dead individuals must meet certain minimum criteria (Table 29–5). In recent years, these criteria have been liberalized because of an increasing organ demand and a severe shortage of available donors.36 Most cardiac donors are younger than age 55 years, although older donors may be used selectively in critically ill recipients or allocated to alternative list recipients. There should be no evidence of severe cardiothoracic trauma or cardiac puncture. An initial echocardiogram is performed to identify significant structural heart disease such as LV hypertrophy or dysfunction, occlusive coronary artery disease, valvular dysfunction, and congenital lesions. Donors with these conditions are typically excluded, although selected marginal organs may be allocated to higher-risk recipients. Angiography is performed to exclude significant coronary artery disease (CAD) in male donors older than age 45 years and in female donors older than age 50 years but may also be performed in younger patients with multiple risk factors for CAD. Patients with active malignancy (excluding nonmelanocytic skin cancers and certain isolated brain tumors) or severe systemic infections are typically excluded. Finally, potential donors are routinely screened for HIV and hepatitis B and C. Although HIV positivity remains an exclusion criterion for organ donation, hepatitis B–and C–positive donors may be allocated to selected higher risk patients or matched to seropositive recipients.
| Age < 55 y |
| Absence of significant structural abnormalities such as: |
| LV hypertrophy (wall thickness >13 mm by echocardiography) |
| Significant valvular dysfunction |
| Significant congenital cardiac abnormality |
| Significant coronary artery disease |
| Adequate physiologic function of donor heart |
| LVEF ≥45% or |
| Achievement of target hemodynamic criteria after hormonal resuscitation and hemodynamic management |
| MAP >60 mm Hg |
| PCWP 8-12 mm Hg |
| Cardiac index >2.4 L/min·m2 |
| CVP 4-12 mm Hg |
| SVR 800-1200 dyne/s·cm5 |
| Dopamine or dobutamine requirement <10 μg/kg/min |
| Negative hepatitis C antibody, hepatitis B surface antigen, and HIV serologies |
| Absence of active malignancy (except nonmelanoma skin cancers and certain primary brain tumors) or overwhelming infection |
After brain death has been determined and the patient has been identified as a potential organ donor, the main goals of organ donor management are to ensure optimal organ function by providing volume resuscitation; optimizing cardiac output; normalizing systemic vascular resistance; maintaining adequate oxygenation; correcting anemia, acid–base, and electrolyte abnormalities; and correcting hormonal imbalances that occur after brain death and that can impair circulatory function. Standardized algorithms incorporating early use of invasive hemodynamic monitoring along with aggressive hemodynamic management and hormonal resuscitation with insulin, corticosteroids, triiodothyronine, and arginine vasopressin have been proposed to improve cardiac donor management and maximize organ utilization, particularly in patients with a LV ejection fraction below 45% on initial echocardiography.36
Most donor hearts are currently harvested locally from the donor by an organ procurement team from the transplant center and transported back to the center for implantation. A cold ischemic period of less than 4 to 6 hours in adult hearts is generally considered optimal. This requirement for short ischemic times leads to the rationale for geographic subdivision into OPOs for cardiac allografts despite the drive for a national list for other organs.
Currently, fewer than 50% percent of potential organ donors in the United States become actual donors.37 Despite ongoing efforts to increase the identification of potential donors, increase the consent rate among eligible donors, expand donor selection criteria, and maximize potential donor organ function, heart transplantation will likely remain a donor-limited field for the foreseeable future.
Donor–recipient matching is performed on the basis of ABO blood group compatibility and overall body size comparability within 20% of body weight. Although the benefit of matching donor organs and recipients with respect to human leukocyte antigen (HLA) has been well established in renal transplantation, HLA prospective crossmatching is reserved for presensitized heart transplant recipients or those with more than 10% to 20% reactivity to a standard panel of common donor antigens. More recently, the use of flow cytometry with recombinant single HLA antigen bead technology has facilitated the prediction of incompatible organs by comparing the recipient’s HLA antibodies with the donor’s HLA type. This “virtual crossmatch“ can eliminate the need for a prospective crossmatch and therefore increase the availability of potential organs, particularly in patients who are sensitized with preformed HLA antibodies as a result of previous pregnancy, transplant, or blood transfusions.
Allocation of thoracic organs in the United States is made according to the recipient’s priority on the United Network for Organ Sharing (UNOS) waiting list and geographic distance from the donor. Priority on the recipient waiting list is determined by a recipient’s assigned status code and time accrued within a status code. In general, patients with the highest medical urgency and lowest expected short-term survival are assigned a higher status code (Table 29–6).
| Status 1A |
| Mechanical circulatory support |
| VAD until clinically stable (≤30 d) or with objective medical evidence of significant device-related complications (thromboembolism, device infection, mechanical failure, or life-threatening ventricular arrhythmias) |
| Total artificial heart |
| IABP |
| ECMO |
| Mechanical ventilation |
| Continuous infusion of single high-dose inotrope or multiple inotropes in addition to continuous hemodynamic monitoring of LV filling pressures |
| Exceptional provision for patients with medical urgency not meeting above criteria |
| Status 1B |
| VAD beyond 30 d |
| Continuous infusion of IV inotropes |
| Status 2 |
| Any candidate not meeting criteria for status 1A or 1B |
Donor hearts are first offered to local Status 1 patients and then extended to Status 1 patients within a 500-mile radius of the donor hospital (Zone A). If no eligible recipients are identified, the organ is offered to local Status 2 patients. This process repeats in a sequence of “zones“ delineated by subsequent concentric circles of 1000- and 1500-mile radii from the donor hospital.
Surgical Technique
The donor heart is explanted, or “harvested,“ by a surgical team at a hospital usually remote from the transplant center, and the procedure needs to be coordinated with the requirements of the surgical teams procuring other organs for transplantation. The donor heart is first arrested with cardioplegic solution. The original surgical technique for orthotopic heart transplantation, or biatrial technique, was originally described by Lower and Shumway in 1960.4 In this procedure, both the donor and recipient hearts are removed by transecting the atria at the midatrial level, leaving the multiple pulmonary venous connections to the left atrium (LA) intact in the posterior wall of the LA, and then transecting the aorta and pulmonary artery just above their respective semilunar valves. The explanted heart is cooled topically by being placed in an iced preservation solution; it is then placed in a secure container and transported expeditiously to the transplant center. Ischemic times average 3 to 4 hours.
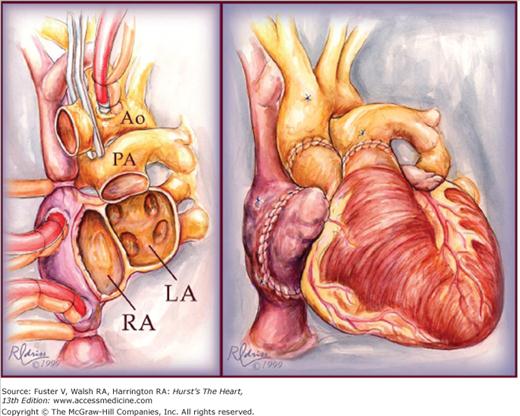
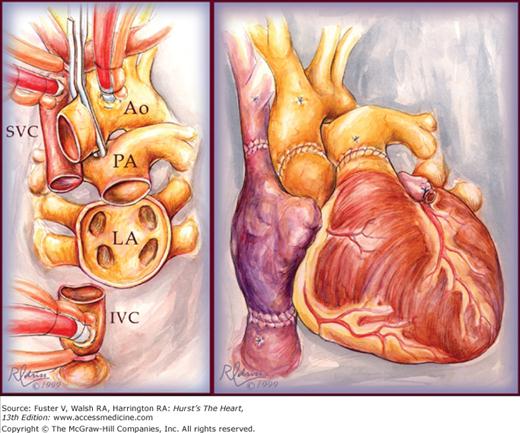
Implantation of the heart in the orthotopic position begins with reanastomosis at the midatrial level, beginning with the atrial septum (Fig. 29–2). Efforts are made to include a generous cuff of donor right atrium so the sinoatrial node will be included. The great vessels are connected just above the semilunar valves. In recent years, the biatrial technique has been modified by leaving the donor atria intact and making anastomoses at the level of the superior and inferior vena cavae and pulmonary veins.38 This bicaval technique (Fig. 29–3) results in less distortion of AV geometry, resulting in improved atrial and ventricular function, less AV valve regurgitation, decreased incidence of atrial arrhythmias, and decreased incidence of donor sinus node dysfunction and heart block requiring permanent pacemaker implantation.39-41
Figure 29–2.
Original biatrial technique for orthotopic heart transplantation. The left panel shows the completed recipient cardiectomy with the recipient atria transected at the midatrial level. The right panel shows the completed re-anastomosis of the donor heart. Ao, aorta; LA, left atrium; PA, pulmonary artery; RA, right atrium. Used with permission from Pahl E, Backer CL, Mavroudis C. Heart transplantation at Children’s Memorial. The Children’s Doctor: Journal of Children’s Memorial Hospital, Chicago. Fall 1999.
Figure 29–3.
Bicaval technique for orthotopic heart transplantation. The leftpanel shows the completed recipient cardiectomy. The recipient atria are completed removed except for except for a cuff of tissue around the pulmonary vein orifices. The superior and inferior vena cavae are transected at their junction with the right atrium. The right panel
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree