Introduction and historical perspective
The prevalence of cardiovascular disease increases with age, and it is estimated that the number of persons older than 65 years in the United States will increase 25–35% over the next 30 years.1 The number of non-cardiac surgical procedures performed in older persons will increase from the current 6 million to nearly 12 million per year, and nearly a fourth of these–major intra-abdominal, thoracic, vascular, and orthopedic procedures–have been associated with significant perioperative cardiovascular morbidity and mortality.2
Guidelines on the assessment and management of perioperative cardiovascular risk for patients undergoing non-cardiac surgery were published by American College of Cardiology/American Heart Association (ACC/AHA) Task Force in 1996 and updated in 2002 and 2006 and revised in 2007.2–4 Guidelines published in 2006 by the ACC/AHA focused the management from non-invasive risk stratification to risk reduction through strategies such as the use of perioperative beta-blockade.
Assessment of preoperative risk for non-cardiac surgery
The range of risk seen in current practice was illustrated in a retrospective study of 663 665 adults with no contraindications to beta-blockers who underwent major non-cardiac surgery in 2000 and 2001 at 329 hospitals in the United States. Orthopedic and abdominal surgery accounted for 70% of cases. In-hospital mortality in patients not treated with beta-blockers increased progressively from 1.4% to 7.4% according to a preoperative assessment of risk using the revised Goldman Cardiac Risk Index5,6 (Level C1).
Eagle et al retrospectively studied 254 consecutive patients presenting to the nuclear cardiology laboratory for dipyridamole-thallium imaging before proposed vascular surgery7 Logistic regression analysis identified five clinical predictors of postoperative cardiac events in patients undergoing major vascular surgery: Q-waves on the ECG, history of angina, ventricular ectopy requiring treatment, diabetes mellitus requiring therapy other than diet, and age above 70 years (Level C1).
A subsequent validation study by Vanzetto added three more clinical predictors to the Eagle criteria which were ischemic ST segment abnormalities on resting ECG, hypertension with severe left ventricular hypertrophy and history of heart failure (HF). This author also identified two independent dipyridamole-thallium test predictors of ischemic events: thallium redistribution and ischemic ECG changes during or after dipyridamole infusion8 (Level C1).
Fleisher and Eagle9 emphasized six factors in a further report, the first five of which are also in the revised Goldman Cardiac Risk Index,6 and are all associated with increased cardiac risk in patients undergoing non-cardiac surgery, including vascular surgery (Level C1).
- Ischemic heart disease (angina or prior MI)
- Heart failure
- High-risk surgery (including intraperitoneal, intratho-racic, and suprainguinal vascular procedures)
- Diabetes mellitus (especially requiring insulin)
- Renal insufficiency
- Poor functional status (defined as the inability to walk four blocks or climb two flights of stairs)
General approach to the patient
Preoperative cardiac evaluation must be carefully tailored to the circumstances that have prompted the consultation and the nature of the proposed surgery. Given an acute surgical emergency, preoperative evaluation might have to be limited to rapid bedside assessment including assessment of vital signs, volume status, basic metabolic panel and electrocardiogram (ECG)2,10 (Class I, Level B). The physician should review available patient data, obtain a history, and perform a physical examination pertinent to the patient’ s problem and the proposed surgery.
History and physical examination
Cardiac conditions such as prior angina, recent or past MI, HF, symptomatic arrhythmias, and history of a pacemaker or implantable cardioverter defibrillator (ICD) should be identified. Modifiable risk factors for coronary heart disease (CHD) should be recorded along with evidence of associated diseases, such as peripheral vascular disease, cerebrovascular disease, diabetes mellitus, renal impairment, and chronic pulmonary disease (Table 66.1).
Table 66.1 Clinical predictors of increased perioperative cardiovascular risk (myocardial infarction, congestive heart failure, death)
Major Unstable coronary syndromes Recent myocardial infarction * with evidence of important ischemic risk by clinical symptoms or non-invasive study Unstable or severe † angina (Canadian class III or IV) Decompensated congestive heart failure Significant arrhythmias High-grade atrioventricular block Symptomatic ventricular arrhythmias in the presence of underlying heart disease Supraventricular arrhythmias with uncontrolled ventricular rate Severe valvular disease Intermediate Mild angina pectoris (Canadian class I or II) Prior myocardial infarction by history or pathologic Q-waves Compensated or prior congestive heart failure Diabetes mellitus Chronic renal insufficiency Minor Advanced age Abnormal electrocardiogram (left ventricular hypertrophy, left bundle branch block, ST-T abnormalities) Rhythm other than sinus (e.g. atrial fibrillation) Low functional capacity (e.g. inability to climb one flight of stairs with a bag of groceries) History of stroke Uncontrolled systemic hypertension |
Reproduced with permission from Eagle et al.2
- Major predictors, when present, mandate intensive management, which may result in delay or cancellation of surgery unless it is urgent.
- Intermediate predictors are well-validated markers of enhanced risk of perioperative cardiac complications and justify careful assessment of the patient’ s current status.
- Minor predictors are recognized markers for cardiovascular disease that have not been proven to independently increase perioperative risk.2
A careful cardiovascular examination should include an assessment of vital signs (including measurement of blood pressure in both arms), carotid pulse, contour and bruits, jugular venous pressure and pattern, auscultation of the lungs, precordial palpation, cardiac auscultation and examination of the extremities for edema and vascular integrity2 The history should also seek to determine the patient’ s functional capacity (Table 66.2).
Table 66.2 Estimated energy requirement for various activities *
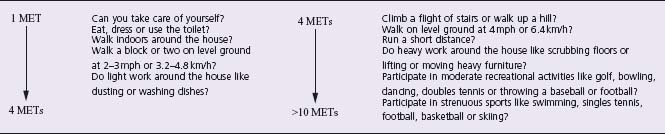
MET, metabolic equivalent. Reproduced with permission from Eagle et al.2 * Adapted from the Duke Activity Status Index and AHA Exercise Standards.
An assessment of an individual’ s capacity to perform a spectrum of common daily tasks has been shown to correlate well with maximum oxygen uptake by treadmill testing10 (Level C).
A patient classified as high risk owing to age or known CAD but who is asymptomatic and runs for 30 minutes daily may need no further evaluation. In contrast, a sedentary patient without a history of cardiovascular disease but with clinical factors that suggest increased perioperative risk may benefit from a more extensive preoperative evaluation (Class II, Level C1).
Management of co-morbid conditions
The stress of non-cardiac surgery may raise heart rate and blood pressure and has been associated with a high incidence of symptomatic and asymptomatic myocardial ischemia in patients with known vascular disease.
Associated conditions often heighten the risk of anesthesia and may complicate cardiac management. The most common of these conditions are discussed below.
If significant pulmonary disease is suspected by history or physical examination, determination of functional capacity, response to bronchodilators, and/or evaluation for the presence of carbon dioxide retention through arterial blood gas analysis may be justified2 (Class I, Level C1).
Management of blood glucose levels in the perioperative period may be difficult. Fragile diabetic patients need careful treatment with adjusted doses or infusions of short-acting insulin based on frequent blood sugar determinations. Historically, it has been acceptable to maintain relatively high glucose levels perioperatively to avoid the attendant risks of hypoglycemic episodes. However, aggressive perioperative glucose control in coronary bypass surgery patients by a continuous, intravenous insulin infusion was superior to intermittent subcutaneous insulin administration in significantly reducing postoperative wound infection.11 Similar benefit may occur surrounding non-cardiac surgery12 (Class I, Level C).
Anemia imposes a stress on the cardiovascular system that may exacerbate myocardial ischemia and aggravate HF13 Preoperative transfusion, when used appropriately in patients with advanced CAD and/or HF, may reduce peri-operative cardiac morbidity (Class II, Level C1). Hematocrits less than 28% are associated with an increased incidence of perioperative ischemia and postoperative complications in patients undergoing prostate and vascular surgery14,15 (Level B).
Due to high risk for the development of further decompensation and death during the perioperative period, patients facing high-stress surgery who have acute coronary syndromes (e.g. unstable angina or decompensated HF of ischemic origin) and stable patients with likelihood of advanced multivessel coronary artery disease, with or without left ventricular dysfunction, will benefit from coronary angiography2 (Class II, Level B). If the non-cardiac surgery is truly urgent, intra-aortic balloon bump counterpulsation as a means of providing short-term myocardial protection in addition to maximal medical therapy4 (Class I, Level C1).
If the patient does not demonstrate unstable symptoms, the identification of known or symptomatic stable CAD or risk factors for CAD can guide the need for further diagnostic evaluation or changes in perioperative management (Class I, Level C). In determining the extent of the preoperative evaluation, it must be remembered that testing should not be performed unless the results would affect perioperative management. These management changes include cancellation of surgery because of prohibitive risk compared with benefit, delay of surgery for further medical management, coronary interventions before non-cardiac surgery, and utilization of an intensive care unit.
Multiple studies have demonstrated an increased incidence of reinfarction after non-cardiac surgery if the prior MI was within 6 months of the operation and especially within 6–12 weeks16,17 (Level C1). Severe hypertension of a chronic nature, e.g. diastolic blood pressure higher than 110 mmHg, should be controlled before any elective non-cardiac surgery.2 The Study of Perioperative Ischemia Research Group trial (POISE), in which patients had continuous perioperative electrocardiographic monitoring, showed that a history of hypertension was one of five independent predictors of postoperative ischemia and one of three independent predictors of increased postoperative mortality.18 Patients with a history of hypertension had almost twice the risk of postoperative myocardial ischemia and almost four times the risk of postoperative death compared with patients without hypertension in the first 48 hours postoperatively19 (Level B).
For patients who present for non-cardiac surgery with signs or symptoms of HF, the goal of the preoperative evaluation should be identification of the underlying disease processes and assessment of the severity of systolic and diastolic dysfunction (Class I, Level B). Ischemic cardiomyopathy is of greatest concern because the patient has a substantial risk for developing further ischemia, leading to myocardial necrosis and potentially a downward spiral. In such patients, a pulmonary artery catheter or intraoperative transesophageal echocardiography may be helpful4 (Level C1). Since both severe stenotic and/or regurgitant valve disease can cause or complicate heart failure, clarification of underlying valve function is important when suspected based on the history of physical exam (Class II, Level C).
The presence of critical aortic stenosis is associated with a very high risk of cardiac decompensation in patients undergoing elective non-cardiac surgery. The presence of any of the classic triad of angina, syncope, and heart failure in a patient with aortic stenosis should alert the clinician to the need for further evaluation and potential interventions, usually valve replacement. However, many patients with severe or critical aortic stenosis may be asymptomatic, and preoperative patients with aortic systolic murmurs warrant a careful history and physical examination and often further evaluation. There are several case series of patients with critical aortic stenosis demonstrating that, when necessary, non-cardiac surgery can be performed with acceptable risk20 (Class I, Level C1).
Decision to undergo diagnostic testing
Several groups have published meta-analyses examining the various preoperative diagnostic tests (Table 66.3). Mantha and colleagues demonstrated good predictive values of ambulatory electrocardiographic monitoring, radionuclide angiography, dipyridamole-thallium imaging, and dobutamine stress echocardiography21 (Level C2). Shaw and co-workers also demonstrated excellent predictive values for both dipyridamole-thallium imaging and dobutamine stress echocardiography22 (Level C1).
Table 66.3 Recommendations for use of ancillary tests in patients undergoing non-cardiac surgery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


