Advantages
Disadvantages
Non invasive
Contraindicated in patients with certain brain aneurysm clips, ferromagnetic shrapnel in eyes, pacemakers and ICDs
No ionizing radiation (useful for serial follow up studies especially in pediatric populations)
ECG and respiratory gating can be challenging creating motion artifacts
No radioactive isotope or iodinated contrast
Acquisition times can be long depending on purpose of study
Images can be acquired in any tomographic plane in 3D without restrictions from body habitus
Patient tolerance (length of scan, claustrophobia, ability to breath hold)
High spatial and temporal resolution
Gd based contrast associated with NSF in patients with low GFR
Many imaging techniques to assess and quantify multiple aspects of cardiac anatomy and function in one scan session
Significant expertise is necessary for a high quality scan (operator dependent) and refined interpretation of results
Fast-paced hardware, software and sequence development improving scanning technology
Increasing number of indications for use
Basic MRI Physics
Can “tune” CMR magnet coils to different frequencies in order to image different paramagnetic nuclei (31P, 23Na, 13C, 1H).
Proton imaging is most commonly used clinically because of high natural abundance in water and lipid molecules (creates high SNR).
In the presence of the static magnetic field (B0), a proportion of the protons will align themselves in the direction of the magnetic field forming a net magnetization.
Size of net magnetization determines the signal intensity that can be used for image formation.
Higher signal intensity means better image quality (hence better image quality at higher field strengths) but also increased susceptibility to certain artifacts.
Protons will “precess” around the axis of B0 and the frequency of precession is dependent on the inherent property of the nuclei being imaged and the field strength.
The angle of alignment with B0 and phase coherence (whether protons are spinning at the same frequency) can be perturbed with radiofrequency (rf) pulse sequences.
Applying controlled rf pulse sequences at different spatial locations allows you to take advantage of the inherent magnetic properties of different tissues (and pathologic states) producing tissue contrast.
After a pulse sequence is applied, the protons in the tissue are “excited” (out of alignment with B0).
As the protons return to baseline alignment (relaxation), the energy released can be recorded by receiving coils and the signal produced can be temporally and spatially encoded to produce an image.
By varying characteristics of the rf pulse sequence (e.g. repetition time, echo time, flip angle etc.) you can create unique pulse sequences and probe tissue characteristics.
This is a unique feature of MRI.
After an rf pulse is applied, the system begins to relax and there are two kinds of relaxation.
T1 relaxation (longitudinal relaxation) is responsible for the realignment of the proton spins with B0 (after the rf pulse has created an angle between the magnetic moment of the proton and B0).
The flip angle is the angle that is generate by the rf pulse between the magnetic moment of the proton and B0.
T1 relaxation is an exponential process that is tissue specific.
The faster the relaxation the shorter the T1 constant (time it takes for the longitudinal magnetization to return to 63 % of its equilibrium value).
Fat has a short T1 and water has a longer T1.
Inflammatory processes will increase T1.
Gd contrast shortens T1.
By taking advantage of intrinsic T1 properties of tissues, you can create pulse sequences that are T1 weighted to bring out tissue contrast and tissue pathology.
T2 relaxation (transverse relaxation or spin-spin relaxation) is the loss of phase coherence of protons after the rf pulse has initially caused them to rotate together.
Immediately after the rf pulse, the spins are said to be “in phase.”
Over time rotating spins will interact with each other and with B0 causing de-phasing.
T2 relaxation is also tissue specific and pathology specific.
T2 is also an exponential process with a time constant that represents the time it takes for the magnetization signal to decay (de-phase) to 37 % of the value immediately after the rf pulse.
Muscle has a short T2 value, but as water content is increased (e.g. edema) the T2 value also increases.
T2* is the time constant (relaxation) that results from spin-spin interactions (T2) as well as magnetic field inhomogeneity.
Although T2 decay is irreversible, the additional decay caused by magnetic field inhomogeneity can be reversed with a 180° refocusing pulse.
Image Acquisition Considerations
Breathing and cardiac motion can create significant motion induced artifacts.
Breathing motion induced artifacts can be mitigated by breath holding.
Typically people can hold their breath for 15–20 s comfortably.
Cardiac motion induced artifacts are minimized by ECG gating.
The system begins acquiring data after a trigger (typically the QRS complex) and subsequent user defined interval (trigger delay).
An acquisition window is set which corresponds to the most quiescent periods of the cardiac cycle (most often diastole).
The wider the acquisition window, the more time there is to acquire data (and thus shortens the total time for the scan) but the more susceptible you are to artifact.
Regular RR intervals are best for data acquisition and MR systems have arrhythmia rejection algorithms to reduced arrhythmia related artifact.
Imaging with irregular heart rates is possible.
Both prospective and retrospective gating is possible.
Averaging acquisitions is a strategy to minimize respiratory motion and to improve SNR but prolongs acquisition time.
Parallel imaging is a technique that shortens acquisition times by taking advantage of the spatial distribution of the receiving coil (usually a coil array). Essentially, the data is “under-sampled” and the missing data is subsequently reconstructed, “filling in” the gaps.
Recent advances have allowed free breathing acquisitions by using a “navigator” that tracks the motion of the diaphragm and only acquires data at the same part of the respiratory cycle.
Eliminates breath holding, but lengthens scan time as images are only acquired when portion of the respiratory cycle is aligned with cardiac cycle.
Basic CMR Techniques
Spin Echo Imaging
Uses slice selective 90° pulse followed by 180° refocusing pulse.
If blood flow is rapid enough for all the blood receiving the first pulse to flow out of the slice, then a signal void is created and images have a “black blood” appearance (Fig. 19.1).
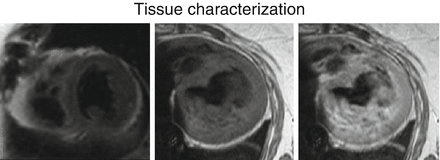
Figure 19.1
Tissue Characterization. Short axis T2 weighted (left) transverse T1 pregadolinium (middle) and transverse T1 post gadolinium (right) black blood MR images of the heart. These sequences are for tissue characterization to assess for myocardial edema, fatty metaplasia, infiltration and inflammation
Slow moving structures (myocardium, blood vessel walls etc.) appear bright.
Provides high contrast between blood and tissue and as such is good for anatomical imaging.
Good for both T1 and T2 weighted images.
Areas of high water content (edema, acute injury) in T2 weighted images appear bright.
Relatively insensitive to magnetic susceptibility artifacts (T2* effects).
Relatively long TR is required which limits image acquisition speed.
Slow blood flow can result in more blood signal (brighter) and poor contrast.
Inconsistent black blood effect can be overcome with black blood preparation schemes (e.g. dual inversion recovery preparation pulse).
Gradient Echo Imaging
Uses a single rf pulse (flip angle usually between 0 and 90°) to generate the signal.
Inflowing blood is fully magnetized and therefore appears bright (“bright blood” imaging).
Myocardium and other structures appear grey (intermediate signal intensity) compared to blood.
Very short TR allows for high temporal resolution making it good for functional imaging.
Used to create cine images to assess cardiac wall motion and function.
Relatively sensitive to magnetic susceptibility artifacts (T2* effects).
Sternal wires and metallic valves can cause artifacts.
Effect can be exploited to assess iron overload in the heart in hemochromatosis.
Turbulent blood flow can cause dephasing and thus give a qualitative estimate of stenotic or regurgitant lesions
Balanced Steady State Free Precession (bSSFP)
Also called FIESTA (GE), TrueFISP (Siemens), or b-FFE (Philips).
Uses a low flip angle, gradient echo pulse sequence.
Signal achieves a steady state.
Has both T1 and T2 weighting (hence “balanced”).
High contrast between blood and myocardium.
Most commonly used sequence for cardiac function but also good enough resolution to provide structural detail.
Phase Contrast (PC) Imaging
Also called velocity encoding.
Can manipulate the MR signal to quantify shifts in the phase of moving spins within a magnetic field yielding information on velocity and direction of movement of the spins.
Can quantify (ml/min) blood flow through an orifice (regurgitant or stenotic) which is useful for
Valve lesions.
Shunt size and shunt fractions (Qp/Qs).
Vascular stenosis (coarctation, pulmonary artery stenosis, bypass grafts).
Magnitude of phase shift that is measured is angle dependent and so slice selection is paramount.
Perfusion Imaging
Myocardial perfusion studies are usually based on imaging the transit of Gd based contrast through cardiac chambers and then through the myocardial perfusion bed.
Most sequences are T1-weighted gradient echo sequences covering multiple slices of the heart at a high temporal resolution.
Also called “first pass” perfusion.
Late Gadolinium Enhancement (LGE)
Image myocardium 10–20 min after administration of Gd using an inversion recovery sequence.
Gd redistributes into the interstitial spaces before renal clearance.
Disease processes that increase extracellular volume (focal infiltrative diseases, fibrosis and scar) will result in increased accumulation of Gd that can be imaged and quantified.
Can assess acute injury patterns (acute MI, myocarditis) as well as chronic injury patterns (chronic infarct, fibrosis).
Transmural extent of infarction is prognostic and predicts functional improvement after revascularization.
The pattern of LGE can help determine the etiology of a cardiomyopathy (Table 19.2).
Table 19.2
Patterns of LGE
Disease
LGE pattern
LGE location
Myocardial Ischemia
Subendocardial with variable transmural extent
Coronary distribution
Myocarditis
Midwall and Subepicardial
Any location though parvovirus has predilection for lateral wall
Chagas
Midwall and Epicardial
Any location though predilection for inferolateral and apex
Non Ischemic Dilated Cardiomyopathy
Midwall
Any location though predilection for septum
Hypertrophic Cardiomyopathy
Midwall
Patchy often worse at thickest portions of LV. RV insertion sites into LV also common locations.
Arrhythmogenic Right Ventricular Cardiomyopathy
Difficult to determine as RV structure is so thin walled
Any location and can extend the entire length of the RV depending on extent of disease.
Sarcoidosis
Any pattern
Any location including RV
Amyloidosis
Typically diffuse, subendocardial
Any location though can be circumferential< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
