Cardiac Diseases Due to Systemic Illness, Genetics, Medication, or Infection
Various systemic illnesses, genetic diseases, chemicals, medications, and sepsis can cause morphologic, functional, and hemodynamic abnormalities of the heart. Cardiac abnormalities include secondary forms of cardiomyopathies (dilated, infiltrative, or restrictive), valvular diseases, pericardial diseases (pericardial effusion, tamponade, or constriction), intracardiac mass, and marantic or Libman-Sacks endocarditis. Infective endocarditis is discussed separately in Chapter 14. Not uncommonly, the echocardiographic detection of a particular cardiac abnormality may be the first diagnostic clue to a systemic or genetic disease, for example, the typical tricuspid and pulmonary valve involvement and appearance in carcinoid and the increased thickness and granular appearance of the ventricular wall in cardiac amyloidosis. Various cardiac manifestations of systemic illnesses frequently seen in the echocardiography laboratory are listed in Table 16-1. The typical echocardiographic findings in these cardiac abnormalities are described in more detail in this chapter, and illustrative examples are given.
Amyloidosis and Infiltrative Cardiomyopathy
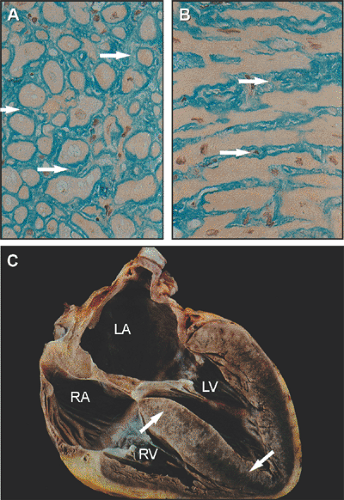
Amyloidosis is due to the deposition of amyloid fibrils in various organs. Amyloid protein is a result of misfolding of extracellular protein, which aggregates in tissues in bundles of β-sheet fibrillar protein (1). It may involve the myocardium, resulting in infiltrative cardiomyopathy (Fig. 16-1). In amyloidosis, myocardial infiltration is primarily interstitial, and the predominant morphologic feature is an increase in myocardial wall thickness without dilatation of the left ventricular (LV) cavity (2) (Fig. 16-2). Ventricular systolic function is not reduced until cardiac amyloidosis reaches the advanced stage, with a marked increase in wall thickness (>15 mm). Although increased wall thickness seen on two-dimensional (2D) echocardiography is the hallmark of cardiac amyloidosis, the diagnosis cannot be excluded when wall thickness is not increased. Amyloid deposits in the heart are diffuse and involve the valves, myocardium, interatrial septum, and pericardium (3). It is common to detect multivalvular regurgitation due to diffuse amyloid deposits in the cardiac valves. Amyloid deposits make the myocardium sparkle on 2D echocardiography; this produces a beautiful image, but the sparkling appearance alone is not diagnostic of cardiac amyloidosis with harmonic imaging because the sparkling appearance is common in patients who do not have cardiac amyloidosis. Other conditions may produce similar echocardiographic features, for example, hypertensive disease (especially in patients with renal failure), glycogen storage disease (4), and hypertrophic cardiomyopathy. Patients with cardiac amyloidosis usually have a low QRS voltage or a pseudoinfarct pattern on the electrocardiogram (ECG) (Fig. 16-3), whereas those with LV hypertrophy, hypertrophic cardiomyopathy, or glycogen storage disease have increased QRS voltages of the LV hypertrophy pattern (Fig. 16-4). Although it can be difficult with 2D echocardiography to differentiate infiltrative cardiomyopathy, such as Fabry disease, from hypertrophic cardiomyopathy or
other forms of LV hypertrophy, the echocardiographic binary appearance of the LV endocardial border, reflecting compartmentalization of endomyocardial glycosphingolipids, may be helpful in diagnosing Fabry disease (5) (Fig. 16-4 right). During an early stage of cardiac amyloidosis, systolic function can be hyperdynamic, and it is not uncommon to see systolic anterior motion of the mitral valve and intracavitary obstruction, as in hypertrophic cardiomyopathy (6). As the disease progresses, however, systolic function gradually deteriorates.
other forms of LV hypertrophy, the echocardiographic binary appearance of the LV endocardial border, reflecting compartmentalization of endomyocardial glycosphingolipids, may be helpful in diagnosing Fabry disease (5) (Fig. 16-4 right). During an early stage of cardiac amyloidosis, systolic function can be hyperdynamic, and it is not uncommon to see systolic anterior motion of the mitral valve and intracavitary obstruction, as in hypertrophic cardiomyopathy (6). As the disease progresses, however, systolic function gradually deteriorates.
Table 16-1 Echocardiographic features of cardiac manifestations of systemic illnesses | ||
|---|---|---|
|
Pulsed wave Doppler echocardiography has been helpful in the evaluation of diastolic abnormalities of cardiac amyloidosis. The initial diastolic abnormality is abnormal relaxation (grade 1 diastolic dysfunction) resulting from increased ventricular wall thickness; the pattern becomes restrictive (grade 3–4 diastolic dysfunction) when progressive amyloid infiltration decreases LV compliance and increases left atrial (LA) pressure (7) (Fig. 16-5). The filling pattern may even normalize temporarily (“pseudonormalized”; grade 2 diastolic dysfunction), as a result of a combined relaxation abnormality and moderate increase in LA filling pressure, before becoming frankly restrictive. Deceleration time (DT) is an important prognostic variable in cardiac amyloidosis (8). The shorter the DT,
the poorer the prognosis. The average survival for patients with a DT of no more than 150 milliseconds is less than 1 year, compared with 3 years when DT is more than 150 milliseconds. Tissue Doppler imaging demonstrates decreased systolic and early diastolic velocities of the mitral anulus in cardiac amyloidosis (Fig. 16-6). Color M-mode of mitral inflow may not show delayed propagation despite a marked abnormality in myocardial relaxation because of the small LV cavity. However, the longitudinal myocardial velocity gradient [(myocardial velocity at the base – myocardial velocity at mid LV)/(distance between two sample sites)] appears to identify patients who have heart failure (9). The strain pattern in cardiac amyloidosis is characteristic, with a marked reduction in myocardial deformation or contraction at the basal segment and even slight lengthening at the mid septal segment (Fig. 16-7).
the poorer the prognosis. The average survival for patients with a DT of no more than 150 milliseconds is less than 1 year, compared with 3 years when DT is more than 150 milliseconds. Tissue Doppler imaging demonstrates decreased systolic and early diastolic velocities of the mitral anulus in cardiac amyloidosis (Fig. 16-6). Color M-mode of mitral inflow may not show delayed propagation despite a marked abnormality in myocardial relaxation because of the small LV cavity. However, the longitudinal myocardial velocity gradient [(myocardial velocity at the base – myocardial velocity at mid LV)/(distance between two sample sites)] appears to identify patients who have heart failure (9). The strain pattern in cardiac amyloidosis is characteristic, with a marked reduction in myocardial deformation or contraction at the basal segment and even slight lengthening at the mid septal segment (Fig. 16-7).
Carcinoid
Carcinoid tumors arise from enterochromaffin cells that are typically in the gastrointestinal tract or lungs. Carcinoid heart disease is caused by a slow-growing metastatic carcinoid tumor, which usually originates in the ileum. Cardiac involvement occurs almost exclusively with liver metastases and is caused by substances released by the tumor, such as serotonin and bradykinin. These substances are responsible for flushing and diarrhea. Because the tumor substances are inactivated by the lung, predominantly the right side of the heart is involved, but the left side may be affected (7% of cases), most likely because a patent foramen ovale allows the tumor substances to pass from the right atrium (RA) to the LA or because of pulmonary metastasis (10). The predominant lesion of cardiac carcinoid is fibrosis of the cardiac valves and endocardium, especially the tricuspid and pulmonary valves. These valvular abnormalities produce characteristic thickening (fibrosis) and restricted motion of the valves that are responsible for severe tricuspid regurgitation, usually mild tricuspid stenosis, and various degrees of pulmonic stenosis (Fig. 16-8). A surgical pathology examination of 75 Mayo Clinic patients showed that all valve dysfunction was related to the presence of carcinoid plaques (Fig. 16-9), which caused thickening and retraction (11). Pure regurgitation was the most common dysfunctional state of the tricuspid valve (80% of cases), mitral valve (97% of cases), and aortic valve (96% of cases). The pulmonary valve was more often stenotic and regurgitant (52% of cases) than purely regurgitant (30% of cases).
In all cases, valve dysfunction was attributed to the presence of carcinoid plaques, which caused both thickening and retraction. Valve thickening is the result of both cellular proliferation (myofibroblasts) and deposition of extracellular matrix (collagen, myxoid, and elastin). These morphologic changes of carcinoid valvular involvement are readily detected with 2D echocardiography (Fig. 16-10). The tricuspid valve is thickened and retracted, with limited mobility, and coaptation is incomplete, resulting in severe tricuspid regurgitation. The severe tricuspid regurgitation produces a pattern of right ventricular (RV) volume overload, with an enlarged RV and ventricular septal motion abnormalities. The pulmonary valve, whose increased thickness and retraction result in pulmonic stenosis, is occasionally difficult to image with transthoracic echocardiography (TTE). The transesophageal longitudinal-axis view of the RV outflow tract may be needed to visualize the pulmonary valve. Characteristically, the wall thickness of the ventricles is normal in carcinoid heart disease, and carcinoid plaque over the endocardial walls in the chambers on the right side may be seen on transesophageal echocardiography (TEE). Doppler recordings from the tricuspid and pulmonic valves are characteristic for severe tricuspid regurgitation and a rapid increase in RV diastolic pressure (Fig. 16-10). The subcostal view is very important in patients with carcinoid. Liver metastases can be identified, and the hepatic vein Doppler recording demonstrates marked systolic flow reversal that
is due to severe tricuspid regurgitation (Fig. 16-11). Also, carcinoid may produce a metastatic tumor embedded in the myocardium (Fig. 16-12), involving the RV in 40% of cases, the LV in 53%, and the ventricular septum in 7% (12). The average size of tumor is 1.8 ± 1.2 cm. The 2D echocardiographic features of carcinoid heart disease are distinctive and readily distinguishable from those of other lesions that produce right-sided heart failure, such as Ebstein anomaly, RV contusion, RV infarct, tricuspid valve dysplasia, and severe tricuspid regurgitation due to intrinsic valvular abnormalities. Rarely, cardiac valvular lesions resembling carcinoid involvement may develop with excessive use of
ergot or a diet pill combination (“fen-phen”). Progression of carcinoid heart disease has been correlated with a higher peak urinary level of 5-hydroxyindoleacetic acid and previous chemotherapy (13).
is due to severe tricuspid regurgitation (Fig. 16-11). Also, carcinoid may produce a metastatic tumor embedded in the myocardium (Fig. 16-12), involving the RV in 40% of cases, the LV in 53%, and the ventricular septum in 7% (12). The average size of tumor is 1.8 ± 1.2 cm. The 2D echocardiographic features of carcinoid heart disease are distinctive and readily distinguishable from those of other lesions that produce right-sided heart failure, such as Ebstein anomaly, RV contusion, RV infarct, tricuspid valve dysplasia, and severe tricuspid regurgitation due to intrinsic valvular abnormalities. Rarely, cardiac valvular lesions resembling carcinoid involvement may develop with excessive use of
ergot or a diet pill combination (“fen-phen”). Progression of carcinoid heart disease has been correlated with a higher peak urinary level of 5-hydroxyindoleacetic acid and previous chemotherapy (13).
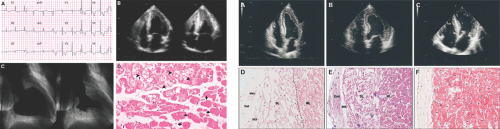 Figure 16-4 Left, characteristic electrocardiogram (A), echocardiogram (B), left ventriculogram (C), and myocardial histologic specimen (D) from a patient with Fabry disease. Arrows, normal cells; arrowheads, cells containing glycolipid inclusion vacuoles. Right, two-dimensional echocardiogram in four-chamber apical view and left ventricular endomyocardial biopsy specimen from two patients with Fabry disease cardiomyopathy (A,D and B,E, respectively) and a patient with hypertrophic cardiomyopathy (C,F). Comparison of the three echocardiographic frames shows the presence of a binary appearance of left ventricular endocardial border in the two Fabry patients (A,B). This echocardiographic finding reflects the glycosphingolipid compartmentalization involving a thickened endocardium (End) with enlarged and engulfed smooth muscle cells (SMC), a subendocardial empty space (SES), and a prominent involvement of subendocardial myocardial layer (SL), while the middle layer (ML) appears partially spared (D,E). The echocardiographic pattern is absent in hypertrophic cardiomyopathy (C), despite a similar thickening of the endocardium (F). (Left, From Chimenti C, Pieroni M, Morgante E, et al. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation, 2004 Aug 31;110:1047–1053 . Epub 2004 Aug 16. Used with permission. Right, From Pieroni et al [5]. Used with permission.) |
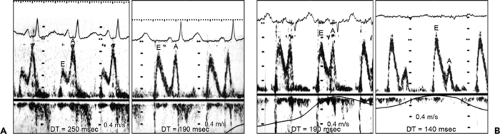 Figure 16-5 Serial mitral inflow Doppler velocities from two patients with cardiac amyloidosis. A: The initial Doppler study (left) showed a relaxation abnormality, with decreased early diastolic filling (E), increased atrial filling (A), and prolonged deceleration time (DT). Six months later (right), the echocardiogram had become pseudonormalized, with progressive cardiac amyloidosis. B: In another patient, the initial study (left) showed a DT of 190 milliseconds, with normal E and A. Seven months later (right), the mitral inflow became typical of a restrictive pattern (increased E, decreased A, and decreased DT), indicating that the initial Doppler pattern was that of “pseudonormalized” inflow. (From Klein et al [7]. Used with permission.) |
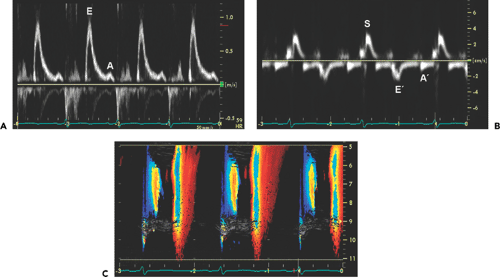 Figure 16-6 Mitral inflow (A), tissue Doppler imaging of the mitral anulus (B), and color M-mode (C
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|