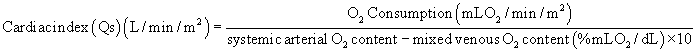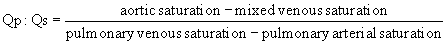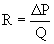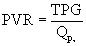CHAPTER 108 Cardiac Catheterization and Fetal Intervention
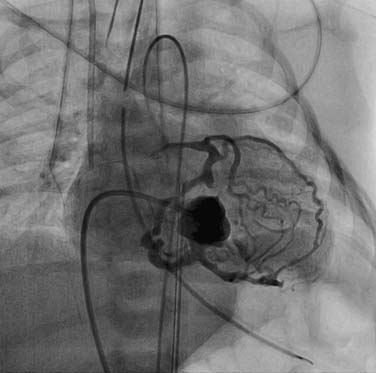
The era of angiographic delineation of cardiac anatomy is fading, although the enormous volume of information provided by this modality has been exhaustively cataloged by Freedom and colleagues.1 Preoperative anatomic information is now routinely and comprehensively acquired by noninvasive imaging. Valvar anatomy is beautifully displayed using transesophageal or three-dimensional (3D) echocardiography. Ventricular function and volumes are quantified using magnetic resonance imaging (MRI). Information regarding anatomic relationships of cardiac and vascular structures can be displayed in a much more user-friendly and intuitively understood format by 3D reconstruction of MRI data than by biplane angiography. Anatomic studies of aortic arch branching in arch anomalies, or of complex venous drainage patterns in heterotaxy syndrome, previously performed in the catheterization laboratory, are now routinely accomplished in the MRI suite.2 Areas where angiography still plays a central imaging role include the branch pulmonary arteries beyond the hilum, and the coronary vasculature. An understanding of anatomic abnormalities of these intricate vascular systems is essential for patient management—for example, in patients with tetralogy of Fallot and multiple aortopulmonary collaterals, or those with pulmonary atresia with intact septum and coronary–cameral fistulae (Fig. 108-1). As MRI and computed tomographic imaging of these lesions improves and is validated, the diagnostic role of angiography will further diminish.3 In this era of interventional catheterization, the most valuable role of angiography will be to provide real-time imaging for guiding intracardiac and intravascular procedures.
Much hemodynamic information can also be derived from echocardiography and MRI, as is discussed in Chapter 107. Although physiologic assessment can be made on estimates based on noninvasive imaging, such as estimating pressure gradients by Doppler echocardiography or estimating flows by MRI, these assessments are still often confirmed in the catheterization laboratory.4 When assessing the advisability of closing defects in older patients with intracardiac shunts, and the potential for prohibitively elevated pulmonary vascular resistance, catheterization to determine the amount of pulmonary flow and to directly measure pulmonary arterial pressures remains the standard approach. The value of this study can also be enhanced by administering nitric oxide to assess the degree of reactivity of the pulmonary vascular bed and to estimate pulmonary resistance in a best-case scenario. For preoperative patients, the operating surgeon’s judgment as to the adequacy or completeness of noninvasively acquired data may influence the decision to perform a catheterization. Thus, knowledge of the advantages and limitations of data gained by all available techniques is critical.
Traditionally, a series of hemodynamic preoperative catheterizations have been a staple of management for palliated patients with single-ventricle disease. Many, possibly most, clinically well patients whose problems have not been identified by adequate echocardiographic imaging are likely to undergo successful bidirectional Glenn operation without excess of morbidity, without the need for a preoperative catheterization.5 The continuing use of hemodynamic catheterization in this setting ultimately depends on the sensitivity of the echocardiogram with regard to problems such as pulmonary arterial distortion or arch obstruction, and on the demonstration of the usefulness of some semi-elective interventions that might occur at preoperative catheterization. The routine scheduling of catheterization before a Glenn or Fontan procedure is likely to receive close scrutiny in the future, and the added value of these studies will need to be defended.
where TPG is the transpulmonary gradient, which is equal to the mean pulmonary artery (PA) pressure minus mean left atrial (LA) pressure, and PVR is pulmonary vascular resistance. The pressures are measured in millimeters of mercury, and flows are typically indexed and expressed in L/min/m2, thus resistance is commonly expressed as mm Hg/L/min/m2, or indexed Wood units.
VASCULAR ACCESS
In some newborns, part or all of the study can be carried out from the umbilical vessels. Simple movements of the arterial catheter are possible, but complex manipulations are limited by the tortuous abdominal course of the catheter. The umbilical vein approach is ideal for balloon atrial septostomy in infants with transposition of the great arteries, and the use of this access site spares the femoral vein from potential injury from large catheters. On the other hand, complex manipulation through obstructed right heart structures from the umbilicus is often time consuming, frustrating, and unsuccessful. Similarly, a percutaneous transhepatic route, if necessitated by occlusion of more traditional venous access routes, may result in complicated catheter courses and prolonged catheterization, but it can be used safely even in infants.6 Rarely, transhepatic access is electively chosen to provide a short, direct course to the atrial septum for defect closure or creation.
INTERVENTIONS
Rashkind and Miller reported the first balloon atrial septostomy in 1966, and it provided a means of palliating the cyanotic child with transposition of the great arteries.7 In the era of neonatal arterial switch operation, early postnatal septostomy allows preoperative stabilization, reduces the need for prostaglandin infusion (with its attendant morbidities), and can be lifesaving in neonates who have inadequate mixing. There has been remarkably little change in the technique or equipment employed since the first description of septostomy. A balloon-tipped catheter, introduced via the umbilical or femoral vein, is positioned in the left atrium through the patent foramen ovale, and it is rapidly accelerated across the septum into the right atrium. Although this was traditionally performed under fluoroscopic guidance in the catheterization laboratory, results have been equally satisfactory with echocardiographic guidance at the bedside in the intensive care unit, with only rare complications.8 Once access is established, the procedure takes only a few minutes, and immediate improvement in systemic saturations can be expected.
Infants presenting for atrial septostomy with a diagnosis of transposition of the great arteries are cyanotic, but they are usually hemodynamically stable on prostaglandin. In contrast, patients with hypoplastic left heart syndrome (HLHS) and restrictive atrial septum are often deeply cyanotic and require ongoing hemodynamic support when they arrive in the catheterization laboratory, despite prostaglandin infusion. Cardiologists and surgeons who care for these infants increasingly recognize the benefit of immediate postnatal left atrial decompression to improve survival.9 When a small preexisting defect can be crossed, a standard pull-through septostomy or blade septostomy can be effective in the short term, but restenosis occurs almost universally.10 Creation of an iatrogenic atrial defect by trans-septal puncture, followed by static balloon dilation or stent placement (or both), is associated with a known incidence of inadvertent cardiac perforation, but it allows a larger, more durable opening of the atrial septum.11,12 Pursuing this aggressive catheter-based approach can result in improved survival rates among patients with this disease, and with the additional potential for fetal intervention, catastrophic neonatal presentation may be averted altogether.9,13
Valvuloplasty
Since Kan and colleagues reported the first static balloon dilation of the pulmonary valve in 1982, this procedure has become widely accepted as the primary intervention for valvar pulmonary stenosis.14 A number of dilating techniques have been applied with good results, and long-term follow-up information is available. The Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry investigators reported excellent results in a large group of pediatric patients with pulmonary stenosis, with 89% of patients achieving gradients of less than 35 mm Hg after the procedure.15,16 To reduce residual gradient, significantly oversized balloons have been used. Experimental induction of right ventricular outflow tract damage and the clinical observation of increased pulmonary insufficiency after oversized balloon dilation support the current practice of limiting the balloon-to-anulus ratio to 1.4.17 Moderately oversized balloons have been safe and effective in reports and in subsequent clinical practice.18,19 Despite dilation with a well-chosen balloon, some patients with dysplastic pulmonary valve do not respond to balloon valvuloplasty, and these patients may require surgical intervention for adequate gradient relief.16 Significant pulmonary insufficiency, occurring in 10% to 40% of patients after percutaneous valvuloplasty, is of little clinical significance in the short term, but longer-term effects of isolated, chronic pulmonary regurgitation have not been studied.20
Pulmonary valvuloplasty techniques developed in older children have been successfully applied to the neonate with critical pulmonary stenosis, and balloon dilation is now the standard for primary neonatal treatment.21,22 Overall complication rates of pediatric pulmonary valve dilation for pulmonary stenosis are extremely low (<1%), but neonates and infants constitute a higher-risk subset of patients.16,21 In neonates with membranous pulmonary atresia and intact ventricular septum who are candidates for right ventricular decompression and biventricular outcome, catheterization may also be pursued as a first step in management. A functionally or truly atretic valve can be probed with a floppy-tipped wire, and, if it proves to be impassable, the valve can then be perforated by puncture with a stiff wire or by application of radiofrequency energy.23,24 After the valve is crossed, serial balloon dilations can eliminate the valvar contribution to right ventricular outflow obstruction, potentially with less resultant pulmonary regurgitation than would be acquired surgically.
The first report of successful balloon dilation of an aortic valve appeared in 1984.25 The procedure is now routinely performed with good results in newborns, infants, and older children. In a large series of 192 procedures, percutaneous aortic valvuloplasty resulted in effective relief of severe aortic stenosis (reduction in peak gradient from 77 to 30 mm Hg).26 Experience has shown that dilation using a balloon inflated to 80% to 100% of the anular dimension of the valve is effective.27 With newer, low-profile balloons, the majority of procedures can be performed from the femoral artery, even in neonates. Freedom from re-intervention has been reported in both surgical and nonsurgical series and is estimated at 50% at 8 years after intervention.28,29 Repeated dilation of the aortic valve in the case of predominantly obstructive disease is possible, and gradient reduction of 50% at the second procedure can be expected.30
Two recent, large single-center experiences with neonatal aortic valvuloplasty have reviewed this procedure comprehensively. The procedure is highly effective, with relative reductions in gradient of 54% and 69% as measured during the procedure.31,32 In the past, this procedure had a significant rate of associated mortality, but this rate has decreased over the nearly 2 decades since the first procedure and is now 4%.32 After retrograde aortic valvuloplasty, femoral pulse loss is common but can be treated with heparin infusion or thrombolytic therapy with some recovery. Femoral arterial injury can be minimized by the application of equally effective antegrade transvenous techniques.26,33
The finding of new aortic regurgitation occurs in approximately 15% to 30% of infants after neonatal valvuloplasty.27,32 Among infants treated primarily with valvuloplasty, a steady increase in the frequency of significant aortic insufficiency has been noted. In a review of 113 infants who were 60 days old or younger at intervention, freedom from moderate or severe aortic regurgitation was 65% at 5 years. In this group, survival free of re-intervention was 48% at 5 years, a rate similar to that noted in earlier, and more heterogeneous series, with many patients presenting for redilation. Factors associated with decreased re-intervention–free survival were investigated, and younger age, higher gradient before and after valvuloplasty, and larger balloon-to-anulus ratio were found to be independent predictors. Of this cohort, 16% needed aortic valve replacement by 5 years.32
Long-term follow-up studies have revealed the potential for catch-up growth of the aortic anulus and left ventricle, with normalization of hypoplastic left heart structures, including the aortic anulus and the left ventricle, observed within the first year of life.31,32
Whereas the aim of pulmonary and aortic valvuloplasty is to create tears along fused commissures to effect gradient relief, mitral valve dilation appears to create a partial tear of the leaflet itself in a manner that creates a greater overall inflow orifice. Rather than leaflet avulsion or anular rupture, disruption of supportive chordal structures poses the greatest risk for major complication during mitral valve dilation. The technique of mitral valvuloplasty to treat patients with rheumatic mitral valve disease was first reported by Lock and associates in 1985.34 Pressed by poor surgical outcomes in young patients with congenital mitral stenosis, Spevak and colleagues attempted balloon angioplasty of the mitral valve in nine patients, predominantly infants and toddlers.35 The procedure resulted in immediate gradient relief in seven of nine patients with heterogeneous valve morphologies. Further exploration of this innovative intervention confirmed the feasibility of achieving short-term hemodynamic benefit by balloon valvuloplasty, and symptomatic improvement among patients was reported after intervention.36 Follow-up over a 2-year period showed only 70% survival at 2 years despite dilation, a rate similar to the 55% predicted survival among similar patients undergoing surgery.36 Contemporary management of severe congenital mitral stenosis continues to employ percutaneous mitral valve dilation, with a 30% reduction in mitral valve gradient. The incidence of significant mitral regurgitation is estimated at 28%, and 5-year survival has improved from 69% in the early experience to 87% more recently.37
Awareness of the deleterious effect of chronic pulmonary insufficiency in many common forms of congenital heart disease after right ventricular outflow tract reconstruction has prompted investigation of transcatheter valve replacement therapies. The most promising first-generation transcatheter valve for the pulmonary position consists of a bovine jugular vein graft combined with a balloon-expandable closed-cell platinum stent.38 The current stent-based design somewhat limits the application of this device to outflow tracts of highly consistent size and shape (i.e., cylinders), so initial human implantations have been almost exclusively in patients with right-ventricle-to-pulmonary-artery conduits. Early experience has been encouraging, with the device functioning to relieve obstruction, in addition to decreasing pulmonary regurgitation as observed angiographically, echocardiographically, and by MRI-derived pulmonary regurgitant fraction (Fig. 108-2). These improvements are sustained in short-term follow-up of nearly 1 year. Perhaps of greatest significance is a demonstrable improvement in New York Heart Association class and measured exercise parameters.39 This technology should evolve and ultimately become an important step in the lifetime management of lesions as common as tetralogy of Fallot.40
Angioplasty
Restenosis rates after surgical repair of coarctation of the aorta have been estimated at 10% to 20% and are associated with age at repair and the degree of arch hypoplasia.41 For patients with isolated recurrent coarctation, transcatheter aortic angioplasty with or without stent implantation has largely supplanted surgical re-intervention. After pioneering work by Sos and colleagues42 and Lock and coworkers43 to test the feasibility of balloon dilation on postmortem or surgically excised specimens, Singer and colleagues44 reported the first successful balloon dilation of recoarctation in a patient in 1982. Kan and coworkers45 described the first seven patients in 1983, and, in 1990, the VACA Registry results showed a reduction in gradient to less than 20 mm Hg in 78% of patients treated.46 Immediate success did not appear to be related to the type of initial surgical procedure.46,47 In 1991, Hijazi and coworkers argued that balloon dilation should be the treatment of choice for recurrent coarctation, based on an 88% success rate and a low rate of complications.48 The field has generally concurred, and recurrent coarctation is now largely managed in the catheterization laboratory.49
Regarding native coarctation, despite the reported efficacy of balloon dilation as a primary intervention, advantages over primary surgical repair are debatable. In older children and adults, the success rates of the procedure, coupled with less frequent occurrence of restenosis and relatively low risk, support balloon angioplasty as a first intervention. Among infants who are less than 3 months old, authors have described favorable results of balloon dilation of native coarctation, with acute gradient reduction from 48 to 8 mm Hg,50 but with restenosis occurring in more than 50% of patients.50–52 Concern over these high restenosis rates, and a 5% to 15% rate of late aneurysms, have precluded widespread acceptance of angioplasty as primary therapy in unoperated infants.53 Therefore, in these younger patients, balloon dilation is pursued primarily as a palliative procedure. Rates of iliofemoral arterial complications, which were high during the early experience with this infant population, have become lower as low-profile dilating balloon catheters have evolved.54
Although the earliest discussion of stent implantation in coarctation described the treatment of severe aortic obstruction, the procedure has proved uniquely effective in treating disease at the other end of the spectrum—mild native or recurrent obstruction.55 In this setting (peak gradient < 20 mm Hg), standard balloon angioplasty may be ineffective because of mild stenoses that may be highly compliant or involve a relatively long segment. Stent placement can allow a well-controlled enlargement of a mildly narrowed aorta over the length of the stent. When gradients as low as 25 mm Hg were reduced to 5 mm Hg, follow-up catheterization showed an associated decrease in left ventricular (LV) end-diastolic pressure, suggesting an improvement in ventricular diastolic function.56 Freedom from re-intervention after stent placement for coarctation is 50% at 5 years, notably higher than in surgical series, reflecting a deliberate staged approach to transcatheter relief of obstruction. This high rate of re-intervention also reflects aggressive treatment of even mild obstruction, with 40% of re-interventions performed in patients with a gradient of 10 mm Hg or less. Overall, pathologic aortic mural injuries such as aneurysm, dissection, or rupture occurred at the time of dilation or stenting in 3 of 153 patients (2%).57
The early experience with pulmonary artery balloon dilation involved low-pressure balloons, and successful dilations were achieved in only 38% to 59% of cases.58–60 Results improved as high-pressure balloons came into use; these provided relief of obstruction in up to 72% of vessels.61 Despite the use of high-pressure balloons, a significant proportion of pulmonary arterial obstructions remained resistant to balloon angioplasty. The new strategy to treat these involves cutting balloons, which are used to initiate controlled vascular injury at the site of resistant lesions; this initial tear can then be extended with standard angioplasty balloons.62 By using coronary cutting balloons on small-vessel pulmonary arterial lesions resistant to high-pressure angioplasty, operators have substantially increased lumen diameter in 92% of previously refractory vessels.63 After the introduction of larger, peripheral cutting balloons, this technology has been applied to larger pulmonary vessels with good effect, although the percentage increase in lumen diameter was less dramatic in these larger vessels.64
Balloon angioplasty of pulmonary vessels is associated with a relatively high rate of complications, some of which are life-threatening. When obstruction to highly stenotic vessels is relieved, distal vessels may be exposed acutely to higher perfusion pressure, resulting in pulmonary edema.65 Direct trauma to the dilation site can create obstructive intimal flaps, contained tears, and vascular rupture.66 Strategies for managing these complications in the catheterization laboratory have been described. Hemorrhage from uncontrolled tears can be controlled by proximal occlusion using embolization coils, whereas endoluminal defects such as flaps can be managed by stent placement.
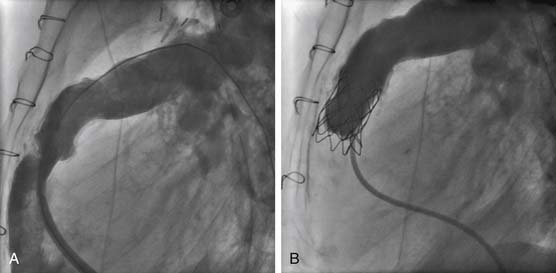
Balloon-expandable stents have been used successfully to relieve pulmonary arterial obstruction since the early 1980s. Stents have been used predominantly in branch pulmonary artery stenoses in larger children, simply because of the size of the available stents, the size of the stent or balloon delivery system, and the risk of iatrogenic restenosis caused by the stent after somatic growth. After experimental evidence that endovascular stents could be safely and effectively redilated, McMahon and associates67 reported on mechanisms of successful redilation in a large group of patients. The recent introduction of more flexible, smaller, and lower-profile stenting systems has resulted in stents being placed more frequently in distal vessels, and in smaller children.68 In the case of early postoperative anastomotic obstruction, stent placement may be preferable to simple angioplasty, as vessel occlusions or stenoses can be opened without employing oversize balloons and risking vessel rupture (Fig. 108-3).69
Transcatheter angioplasty techniques have also proved valuable in extending the life of homograft conduits used in right ventricular outflow tract reconstruction. These grafts can become obstructive and require replacement because of contraction, kinking, neointimal “peel” accumulation, external compression, or simply patient growth. Standard balloon angioplasty rarely provides definitive relief. However, balloon expandable stent placement can decrease the need for surgical re-intervention.70,71 In a review of a single-center large study of 221 patients who had bare stents implanted in right ventricle to pulmonary artery (PA) conduits between 1990 and 2004, acute hemodynamic changes after stenting included significantly decreased right ventricular (RV) systolic pressure and peak right-ventricle-to-PA gradient. Surgical removal of a malpositioned stent was required in five cases. Stents could be redilated, and at subsequent catheterization, additional stents could be placed. By Kaplan-Meier analysis, median freedom from conduit surgery after stenting was 2.7 years. Younger age, smaller conduit diameter, and higher ratio of RV to aortic pressure were associated with shorter freedom from surgery.72
Systemic venous obstruction is a well-recognized complication requiring re-intervention in patients after atrial switch procedures. In both cardiac and noncardiac patient populations, systemic venous obstruction occurs with increasing frequency as a result of chronic indwelling catheters, and, in some cases, cannulation for extracorporeal membrane oxygenation. Both angioplasty and stent placement have been applied successfully in the setting of superior vena cava obstruction for patients with and without congenital heart disease.73 The superiority of stent placement over simple balloon angioplasty with regard to gradient relief and durability of result is suspected but has not been firmly established.
Pulmonary vein stenosis that occurs de novo or after repair of total anomalous pulmonary venous return remains a disease that is untreatable in the catheterization laboratory. On the basis of favorable results after balloon dilation angioplasty of congenital lesions, Driscoll and colleagues attempted pulmonary vein dilation in the early 1980s, and they observed early restenosis associated with clinical decline.74 Endovascular stents applied to pulmonary venous obstruction nearly a decade later failed to alter the course of the progressive and intractable reobstruction, which rapidly redeveloped within months of intervention.75,76 Stent modifications—such as the application of a physical barrier to intimal ingrowth (e.g., a covered stent) or drug-eluting properties—hold some promise, but results are as yet unreported.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree