The aim of this study was to evaluate the role of left ventricular (LV) dysfunction (global and regional, systolic and diastolic) acute dyssynchrony, ischemic mitral regurgitation (MR), and afterload changes in acute hypertensive pulmonary edema (AHPE). Forty-four consecutive patients were evaluated by comprehensive echocardiography during clinical and radiologic pulmonary edema (63 ± 29 minutes after first dose of treatment) and after 48 to 92 hours. Twenty age- and gender-matched asymptomatic hypertensive and diabetic subjects served as controls. AHPE was associated with increased afterload (estimated arterial elastance 3.0 vs 2.3 mm Hg/ml, p = 0.024) and subsequent decreased longitudinal LV systolic function (mean strain of 6 basal segments −11.0% vs −15.4%; p = 0.015) compared to the stable follow-up state. However, global LV systolic function was maintained (estimated ventricular elastance 1.7 vs 1.6 mm Hg/ml, stroke work 76.7 vs 84.5 cJ, ejection fraction 0.33 vs 0.37, all nonsignificant). Except for diastolic filling time (ratio to cardiac cycle 0.41 vs 0.49, p <0.001), other indexes of diastolic function, dyssynchrony, and MR severity were similar between evaluations. Patients with AHPE had worse ventricular–arterial coupling, systolic function, estimated diastolic stiffness, and filling pressures compared to asymptomatic controls, suggesting a decreased capacity to adapt to changes in loading. In conclusion, acute alterations of systolic and diastolic LV function, myocardial synchrony, and ischemic MR are unlikely mechanisms of AHPE. Rather, AHPE is likely to develop in patients with decreased systolic and diastolic capacity to adapt to acute changes in loading.
Acute heart failure is the first cause of hospital admission in patients >65 years of age. It carries a poor prognosis, with a mortality of 20% at 1 year. Acute pulmonary edema represents 1/6 of all forms of acute heart failure and is frequently associated with hypertension crisis (acute hypertensive pulmonary edema [AHPE]). Despite its high prevalence and severity, remarkably few studies have evaluated cardiac function during AHPE, and these used only conventional echocardiographic methods and simple functional parameters. Acute systolic left ventricular (LV) dysfunction was thought to play a dominant role in the pathogenesis of AHPE, but LV ejection fraction was unchanged during AHPE compared to 24 to 72 hours afterward. However, LV ejection fraction is load-dependent and weak measurement of LV function in acute heart failure. Acute diastolic dysfunction, transient myocardial ischemia, ischemic mitral regurgitation (MR), acute alteration of ventricular–arterial coupling, and acute myocardial dyssynchrony have been proposed as potential mechanisms of AHPE, but no detailed studies have evaluated these hypotheses. We set up this study to assess the role of all these proposed mechanisms in the pathogenesis of AHPE.
Methods
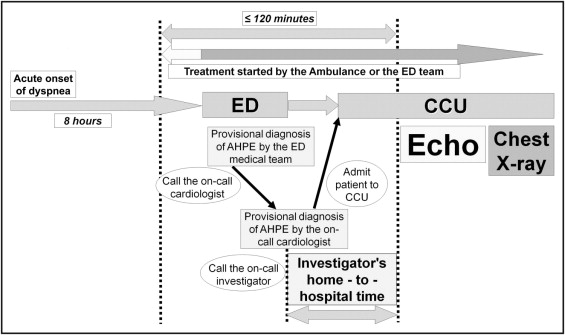
Over a period of 12 months, 86 consecutive patients admitted to the cardiac care unit of our hospital with a clinical diagnosis of AHPE and without known severe valvar disease or evidence of ST-segment elevation acute coronary syndrome were first screened by an on-call cardiologist ( Figure 1 ) . Eligible patients for screening were those presenting with (1) acute onset of dyspnea within the preceding 8 hours, (2) pulmonary rales, (3) systolic blood pressure ≥160 mm Hg at admission, and (4) sinus rhythm. When these criteria were met, the on-call cardiologist (if different from the investigators) called 1 of the 3 investigators (A.D.M., R.C.R., M.F.) who started the first echocardiographic study within 120 minutes from the beginning of any intravenous treatment. For ethical reasons, treatment was given without delay in the ambulance or by the emergency department team. Patients requiring assisted mechanical ventilation were not studied. All patients were still dyspneic and in pulmonary edema by the time the first echocardiographic study was completed, and chest x-ray confirmation of pulmonary congestion was performed after echocardiographic assessment. Only patients in whom pulmonary edema was confirmed by chest x-ray were included; 3 patients were excluded because they did not have AHPE (1 had exacerbation of chronic obstructive pulmonary disease, 1 had consolidation on chest x-ray, and 1 had acute lung injury during sepsis). Patients were subsequently excluded from the study if they had any of the following conditions: (1) acute myocardial infarction confirmed by a creatine kinase-MB or troponin I level >99th percentile of the upper reference limit (n = 15), (2) greater than moderate left-sided valvar disease (n = 12), (3) death (n = 1) or discharge before 48 hours (n = 4), and (4) use of inotropic drugs during the acute event (n = 7). Thus, the final study population consisted of 44 patients.
The institutional ethics committee of the University and Emergency Hospital of Bucharest reviewed the protocol and approved the study. The committee issued a partial waiver regarding the informed consent form during the acute episode, which was considered clinically indicated. All patients gave written informed consent before the second echocardiographic study.
A detailed description (including additional bibliographic references) of the echocardiographic protocol and measured parameters is given in the Supplementary Methods . In brief, 2 detailed echocardiographic studies were performed for each patient, during the acute episode and 48 to 96 hours afterward, when the patient had no signs or symptoms of pulmonary edema; the same investigator acquired the 2 studies according to a standardized protocol. Digital loops of ≥3 cardiac cycles were acquired and stored using a Vivid I system (General Electric, Milwaukee, Wisconsin) equipped with a 1.5- to 4.0-MHz probe. Off-line measurement and analysis were performed using dedicated software (Echopac 7.0.0, General Electric) for all acquisitions by 1 investigator (A.D.M.) who was blinded to all clinical data. All measurements are reported as an average of 3 consecutive beats.
Standard measurements of wall thickness and chamber diameters were made according to current recommendations. Global LV systolic function was assessed by indexed cardiac output, stroke work, cardiac power, +dP/dt, isovolumic acceleration, LV outflow tract acceleration, LV ejection fraction, LV fractional shortening, and Tei index. Arterial elastance and ventricular elastance were estimated using validated noninvasive methods; their ratio was used to assess ventricular–arterial coupling. Global LV diastolic function was assessed according to current recommendations from the mitral flow profile and by estimating LV filling pressures. The ratio between peak early velocity of diastolic mitral flow/mean velocity of mitral annular motion in the longitudinal axis during early diastolic filling and LV end-diastolic volume was used as a surrogate for LV end-diastolic stiffness. We also measured diastolic filling time (ratio to cardiac cycle), A wave duration, and left atrial ejection fraction. Longitudinal LV function was assessed by tissue Doppler derived indexes. Six mitral annular sites were sampled in order to calculate mean velocities (systolic, s′; early diastolic, e′; and atrial, a′), by averaging the corresponding peak on-line longitudinal velocities. Off-line analysis was used to measure mean peak displacement (D) of 6 annular LV sites, and mean peak strain, and strain rate of 6 basal and 12 LV segments (6 basal and 6 midventricular). We also assessed indexes of LV twist, untwist, and torsion (by 2-dimensional speckle-tracking echocardiography); intra- and interventricular dyssynchrony indexes; myocardial ischemia (by the presence of regional wall motion abnormalities or abnormal postsystolic shortening); severity of MR; right ventricular function; and pulmonary vascular resistance. A control group of 20 asymptomatic patients with hypertension and type 2 diabetes (matched for age and gender) was recruited from an ongoing study performed in our institution ( http://clinicaltrials.gov , identifier NCT00980187 ; present study identifier NCT00829855 ). Data from these patients were recorded and analyzed as described earlier.
We previously reported in detail the reasonable intraobserver variability of conventional and tissue Doppler velocity measurements (coefficient of variation <20%). For this study we assessed the intraobserver variability of time to peak systolic myocardial velocity used for calculating dyssynchrony indexes (coefficient of variation 7%), tissue Doppler-derived strain and strain rate (coefficients of variation 14% and 15% respectively), and all parameters of assessing LV torsional mechanics (coefficients of variation 15% to 20%) by repeating 25 pairs of random measurements from the main study group. Cohen kappa coefficient was used to report agreement in interpreting LV wall motion abnormalities (kappa 0.63) and presence of abnormal postsystolic shortening (kappa 0.44).
Statistical analysis was performed with SPSS 14.0 (SPSS, Inc., Chicago, Illinois) and GraphPad InStat 3.0 (GraphPad, La Jolla, California). Results are presented as mean ± SD. Paired-samples t test was used for comparison of mean values with normal distribution and Wilcoxon signed-rank test for comparison of values non-normally distributed. Comparisons between categorical values were computed by chi-square test. Correlations between independent variables are reported using the Pearson correlation coefficient. A p value <0.05 for a 2-tailed test was considered statistically significant. One prespecified subgroup analysis was performed for patients with preserved LV ejection fraction (≥45%).
Results
General characteristics of the study group are presented in Table 1 . On admission 9 patients (20%) had left bundle branch block, which persisted at follow-up. Systolic blood pressure at admission was 196 ± 31 mm Hg; all patients had long-term hypertension. Because the initial echocardiographic study was performed 63 ± 29 minutes after initiation of treatment, mean blood pressure was similar between the 2 echocardiographic evaluations.
| Study Group | Controls | p Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 69 ± 11 | 66 ± 6 | 0.33 |
| Men | 17 (39%) | 8 (40%) | 0.57 |
| Body mass index (kg/m 2 ) | 28.1 ± 4.2 | 28.6 ± 3.6 | 0.68 |
| Medical history | |||
| Coronary artery disease | 20 (45%) | 0 | — |
| Myocardial infarction | 16 (36%) | 0 | — |
| Coronary revascularization | 2 (5%) | 0 | — |
| Stable angina pectoris | 8 (18%) | 0 | — |
| Ischemic stroke | 7 (16%) | 0 | — |
| Diabetes mellitus | 20 (45%) | 20 (100%) | — |
| Peripheral vascular disease | 4 (9%) | 0 | — |
| Chronic heart failure | 16 (36%) | 0 | — |
| Previous acute pulmonary edema | 13 (30%) | 0 | — |
| Drug treatment during acute phase | |||
| Furosemide | 44 (100%) | — | — |
| Nitroglycerin | 43 (98%) | — | — |
| Opiates | 16 (36%) | — | — |
| Other antihypertensives (intravenous enalaprilat, sublingual captopril, oral clonidine, sublingual nifedipine) | 4 (9%) | — | — |
| Drug treatment at follow-up | |||
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 37 (84%) | 15 (75%) | 0.30 |
| β Blockers | 32 (73%) | 9 (45%) | 0.032 |
| Calcium channel blockers | 20 (45%) | 6 (30%) | 0.19 |
| Long-acting nitrates | 23 (52%) | 0 | <0.001 |
| Statins | 27 (61%) | 14 (70%) | 0.35 |
Cardiac dimensions and volumes were similar between evaluations. Afterload was increased and LV longitudinal systolic function and basal LV rotation were decreased during AHPE compared to the stable follow-up state; however, global LV systolic function and LV apical rotation and twist were unchanged ( Table 2 ). These modifications were independent of heart rate (R 2 <0.03, p >0.12 for all correlations). Diastolic filling time was decreased during AHPE compared to follow-up, mainly related to increased heart rate (R = 0.59, R 2 = 0.34, p <0.001). All other parameters of systolic and diastolic LV function were not significantly different between evaluations. Dyssynchrony indexes and markers of myocardial ischemia were not different between evaluations. MR was present in 16 patients (36%) during AHPE, but MR severity and geometry of the mitral valve apparatus was similar to follow-up. Right ventricular function was preserved during AHPE compared to follow-up ( Table 2 ).
| Patients | Acute Episode | Follow-Up | p Value | |
|---|---|---|---|---|
| General parameters | ||||
| Heart rate (beats/min) | 44 | 92 ± 19 | 74 ± 13 | <0.001 |
| Mean blood pressure (mm Hg) | 44 | 104 ± 19 | 102 ± 16 | 0.61 |
| Dimensions and volumes | ||||
| Left atrial volume (ml) | 44 | 82.0 ± 30.2 | 89.4 ± 28.5 | 0.24 |
| Ventricular septum thickness (mm) | 44 | 12.9 ± 2.9 | 12.9 ± 3.4 | 0.98 |
| Left ventricular posterior wall thickness (mm) | 44 | 12.0 ± 3.2 | 12.0 ± 2.9 | 0.97 |
| Left ventricular end-diastolic diameter (mm) | 44 | 48.4 ± 9.8 | 48.7 ± 8.9 | 0.91 |
| Left ventricular end-diastolic volume (ml) | 44 | 124 ± 61 | 134 ± 67 | 0.46 |
| Right atrial diameter (mm) | 43 | 39.3 ± 7.4 | 40.5 ± 8.3 | 0.48 |
| Right atrial volume (ml) | 44 | 50.9 ± 20.4 | 55.4 ± 23.0 | 0.33 |
| Right ventricular diameter (mm) | 43 | 33.2 ± 4.8 | 33.8 ± 4.7 | 0.55 |
| Global left ventricular systolic function | ||||
| Indexed cardiac output (L/min/m 2 ) | 42 | 2.6 ± 1.0 | 2.4 ± 0.8 | 0.25 |
| Stroke work (cJ) | 42 | 76.7 ± 30.6 | 84.5 ± 29.9 | 0.24 |
| Cardiac power (W) | 42 | 1.1 ± 0.5 | 1.0 ± 0.4 | 0.19 |
| +dP/dt (mm Hg/s) | 9 | 961 ± 276 | 1,030 ± 235 | 0.57 |
| Isovolumic acceleration (m/s 2 ) | 39 | 1.3 ± 0.9 | 1.1 ± 0.5 | 0.09 |
| Left ventricular outflow tract acceleration (m/s 2 ) | 43 | 15.1 ± 5.0 | 15.7 ± 5.0 | 0.57 |
| Ejection fraction | 43 | 0.33 ± 0.14 | 0.37 ± 0.14 | 0.28 |
| Fractional shortening | 43 | 0.20 ± 0.09 | 0.21 ± 0.12 | 0.57 |
| Tei index | 41 | 0.54 ± 0.16 | 0.52 ± 0.16 | 0.63 |
| Ventricular elastance (mm Hg/ml) | 40 | 1.67 ± 0.85 | 1.56 ± 0.43 | 0.87 |
| Arterial elastance (mm Hg/ml) | 42 | 3.03 ± 1.79 | 2.30 ± 0.71 | 0.024 |
| Arterial elastance/ventricular elastance ratio | 40 | 1.92 ± 1.02 | 1.58 ± 0.65 | 0.02 |
| Diastolic left ventricular function | ||||
| E wave (cm/s) | 44 | 68 ± 27 | 72 ± 24 | 0.52 |
| A wave (cm/s) | 40 | 76 ± 27 | 80 ± 32 | 0.57 |
| E wave/A wave ratio | 40 | 1.0 ± 0.6 | 1.2 ± 0.9 | 0.22 |
| E wave deceleration time (ms) | 38 | 198 ± 83 | 223 ± 72 | 0.17 |
| Isovolumic relaxation time (ms) | 44 | 108 ± 37 | 103 ± 29 | 0.54 |
| -dP/dt (mm Hg/s) | 5 | 698 ± 175 | 667 ± 73 | 0.72 |
| Mitral flow propagation velocity, Vp (cm/s) | 44 | 31 ± 10 | 31 ± 11 | 0.88 |
| Mean peak velocity of mitral annular motion in longitudinal axis during early diastolic filling, e′ (cm/s) | 44 | 4.9 ± 1.6 | 4.8 ± 1.5 | 0.23 |
| Mean peak velocity of mitral annular motion in longitudinal axis during atrial contraction, a′ (cm/s) | 43 | 7.0 ± 2.6 | 6.8 ± 2.4 | 0.74 |
| E wave/mean velocity of mitral annular motion in longitudinal axis during early diastolic filling, E/e′ ratio | 43 | 17.6 ± 8.9 | 18.5 ± 9.4 | 0.66 |
| (E wave/mean velocity of mitral annular motion in longitudinal axis during early diastolic filling)/left ventricular end-diastolic volume ratio | 44 | 0.17 ± 0.13 | 0.17 ± 0.14 | 0.91 |
| E wave/mitral flow propagation velocity, E/Vp ratio | 44 | 2.3 ± 0.8 | 2.4 ± 0.8 | 0.34 |
| Duration of systole (ms) | 43 | 293 ± 68 | 311 ± 55 | 0.18 |
| Diastolic filling time (ratio to cardiac cycle) | 43 | 0.41 ± 0.1 | 0.49 ± 0.1 | <0.001 |
| A wave duration (ms) | 44 | 138 ± 27 | 139 ± 26 | 0.84 |
| Left atrial ejection fraction | 44 | 0.34 ± 0.14 | 0.38 ± 0.10 | 0.15 |
| Longitudinal left ventricular systolic function | ||||
| Mean peak annular systolic velocity, s′ (cm/s) | 44 | 5.6 ± 1.8 | 5.5 ± 1.6 | 0.86 |
| Mean peak systolic displacement of mitral annulus, D (mm) | 44 | 5.6 ± 1.9 | 6.4 ± 1.7 | 0.029 |
| Mean basal strain (6 segments) (%) | 43 | −11.0 ± 8.9 | −15.4 ± 7.3 | 0.015 |
| Mean basal strain rate (6 segments) (1/s) | 44 | −1.1 ± 3.0 | −1.1 ± 0.7 | 0.92 |
| Mean strain (12 segments) (%) | 43 | −12.3 ± 4.8 | −14.8 ± 4.8 | 0.018 |
| Mean strain rate (12 segments) (1/s) | 44 | −1.2 ± 1.8 | −1.2 ± 0.4 | 0.76 |
| Left ventricular torsion and untwist | ||||
| Basal left ventricular rotation (°) | 18 | −3.8 ± 2.2 | −5.5 ± 3.1 | 0.05 |
| Apical left ventricular rotation (°) | 18 | 4.2 ± 5.3 | 3.9 ± 5.5 | 0.87 |
| Left ventricular twist (°) | 18 | 7.8 ± 5.0 | 9.3 ± 5.5 | 0.39 |
| Time to peak twist (ratio of corresponding systole) | 18 | 0.85 ± 0.34 | 0.86 ± 0.29 | 0.95 |
| Twist rate (°/s) | 16 | 73 ± 27 | 70 ± 26 | 0.75 |
| Untwist rate (°/s) | 18 | −63 ± 33 | −65 ± 31 | 0.91 |
| Time from aortic valve closure to untwist rate (ms) | 15 | 100 ± 74 | 105 ± 104 | 0.90 |
| Left ventricular torsion (°/cm) | 18 | 10.5 ± 6.4 | 13.1 ± 7.8 | 0.29 |
| Dyssynchrony indexes | ||||
| Maximal difference between time to peak annular systolic velocity (ms) | 44 | 60.6 ± 44.1 | 77.6 ± 48.7 | 0.09 |
| Maximal difference between time to peak systolic displacement (ms) | 44 | 47.4 ± 42.2 | 40.1 ± 35.9 | 0.40 |
| Yu index (ms) | 44 | 34.8 ± 20.1 | 38.5 ± 20.9 | 0.41 |
| Aortic preejection time (ms) | 42 | 89 ± 24 | 87 ± 20 | 0.74 |
| Aortic–pulmonary delay (ms) | 44 | 19 ± 48 | 18 ± 44 | 0.92 |
| Myocardial ischemia | ||||
| Wall motion score index | 44 | 2.3 ± 0.9 | 2.2 ± 0.9 | 0.71 |
| Number of segments with postsystolic shortening (16-segment model) | 44 | 5.9 ± 5.0 | 6.6 ± 5.6 | 0.55 |
| Mitral regurgitation | ||||
| Vena contracta (mm) | 5 | 2.6 ± 1.5 | 3.5 ± 1.4 | 0.34 |
| Mitral regurgitation jet area (cm 2 ) | 16 | 3.7 ± 2.8 | 3.9 ± 3.1 | 0.78 |
| Mitral regurgitation jet area/left atrial area ratio | 16 | 0.14 ± 0.09 | 0.16 ± 0.13 | 0.76 |
| Mitral regurgitation volume (ml/s) | 4 | 64 ± 13 | 77 ± 35 | 0.50 |
| Tenting area (cm 2 ) | 43 | 2.6 ± 1.0 | 3.4 ± 5.3 | 0.29 |
| Mitral annular diameter (mm) | 43 | 30 ± 5 | 30 ± 6 | 0.99 |
| Right ventricular function | ||||
| Tricuspid annular peak systolic excursion (mm) | 38 | 21.1 ± 4.4 | 20.2 ± 2.5 | 0.35 |
| Peak annular systolic velocity (cm/s) | 43 | 14.5 ± 4.6 | 12.8 ± 3.9 | 0.06 |
| Right ventricular isovolumic acceleration (m/s 2 ) | 38 | 260 ± 127 | 205 ± 144 | 0.08 |
| Basal strain (%) | 31 | −26.5 ± 15.6 | −27.6 ± 9.3 | 0.74 |
| Basal strain rate (1/s) | 30 | 2.7 ± 1.1 | 2.3 ± 0.9 | 0.12 |
| Trans tricuspid gradient (mm Hg) | 30 | 21 ± 12 | 16 ± 11 | 0.11 |
| Pulmonary vascular resistance (dyne×s×cm 5 ) | 26 | 151 ± 85 | 120 ± 43 | 0.10 |
Eleven patients (25%) had preserved LV ejection fraction (i.e., ≥45%) during AHPE ( Table 3 ). These patients had LV concentric remodeling (mean relative wall thickness 0.69 ± 0.15, range 0.46 to 1.00). LV hypertrophy (LV mass index >125 g/m 2 ) was present in 7 patients (64%). Main measured parameters did not differ significantly between echocardiographic evaluations. Left bundle branch block was absent in this subgroup.
| Patients | Acute Episode | Follow-Up | p Value | |
|---|---|---|---|---|
| General parameters | ||||
| Heart rate (beats/min) | 11 | 82 ± 24 | 67 ± 12 | 0.08 |
| Mean blood pressure (mm Hg) | 11 | 101 ± 15 | 110 ± 14 | 0.19 |
| Dimensions and volumes | ||||
| Left atrial volume (ml) | 11 | 92.8 ± 23.7 | 86.1 ± 21.5 | 0.50 |
| Ventricular septum thickness (mm) | 11 | 15.1 ± 3.3 | 15.1 ± 3.2 | 0.98 |
| Left ventricular posterior wall thickness (mm) | 11 | 14.3 ± 2.4 | 13.6 ± 2.0 | 0.48 |
| Left ventricular end-diastolic diameter (mm) | 11 | 42.0 ± 3.5 | 43.6 ± 4.2 | 0.34 |
| Left ventricular end-diastolic volume (ml) | 11 | 74.9 ± 21.5 | 84.6 ± 16.5 | 0.25 |
| Right atrial diameter (mm) | 11 | 38.1 ± 4.8 | 39.4 ± 4.3 | 0.56 |
| Right atrial volume (ml) | 11 | 49.6 ± 15.8 | 57.4 ± 14.6 | 0.24 |
| Right ventricular diameter (mm) | 11 | 31.1 ± 3.4 | 32.2 ± 5.3 | 0.56 |
| Global left ventricular systolic function | ||||
| Indexed cardiac output (L/min/m 2 ) | 11 | 5.4 ± 2.3 | 4.9 ± 1.6 | 0.53 |
| Stroke work (cJ) | 11 | 92.5 ± 27.5 | 105.5 ± 20.2 | 0.36 |
| Cardiac power (W) | 11 | 1.2 ± 0.6 | 1.2 ± 0.4 | 0.83 |
| Isovolumic acceleration (m/s 2 ) | 11 | 1.5 ± 1.1 | 1.2 ± 0.6 | 0.40 |
| Left ventricular outflow tract acceleration (m/s 2 ) | 11 | 18.9 ± 6.3 | 18.2 ± 6.0 | 0.80 |
| Ejection fraction | 11 | 0.53 ± 0.08 | 0.53 ± 0.08 | 0.89 |
| Fractional shortening | 11 | 0.28 ± 0.08 | 0.34 ± 0.07 | 0.07 |
| Tei index | 10 | 0.51 ± 0.14 | 0.47 ± 0.10 | 0.42 |
| Ventricular elastance (mm Hg/ml) | 11 | 1.69 ± 0.35 | 1.92 ± 0.37 | 0.24 |
| Arterial elastance (mm Hg/ml) | 11 | 2.37 ± 0.82 | 2.25 ± 0.51 | 0.67 |
| Arterial elastance/ventricular elastance ratio | 11 | 1.42 ± 0.54 | 1.18 ± 0.24 | 0.15 |
| Diastolic left ventricular function | ||||
| E wave (cm/s) | 11 | 75 ± 22 | 77 ± 20 | 0.81 |
| A wave (cm/s) | 11 | 83 ± 28 | 86 ± 26 | 0.82 |
| E wave/A wave ratio | 11 | 1.0 ± 0.4 | 1.0 ± 0.5 | 0.92 |
| E-wave deceleration time (ms) | 11 | 269 ± 93 | 253 ± 84 | 0.68 |
| Isovolumic relaxation time (ms) | 11 | 108 ± 27 | 103 ± 17 | 0.61 |
| Mitral flow propagation velocity, Vp | 11 | 38 ± 8 | 41 ± 10 | 0.41 |
| Mean peak velocity of mitral annular motion in longitudinal axis during early diastolic filling, e′ (cm/s) | 11 | 4.7 ± 1.1 | 4.9 ± 1.7 | 0.73 |
| Mean peak velocity of mitral annular motion in longitudinal axis during atrial contraction, a′ (cm/s) | 11 | 7.3 ± 2.3 | 7.3 ± 2.1 | 0.94 |
| E wave/mean velocity of mitral annular motion in longitudinal axis during early diastolic filling, E/e′ ratio | 11 | 20.0 ± 10.3 | 22.0 ± 13.0 | 0.69 |
| (E wave/mean velocity of mitral annular motion in longitudinal axis during early diastolic filling)/left ventricular end-diastolic volume ratio | 11 | 0.28 ± 0.14 | 0.27 ± 0.16 | 0.86 |
| E wave/mitral flow propagation velocity, E/Vp ratio | 11 | 2.0 ± 0.6 | 2.0 ± 0.5 | 0.70 |
| Duration of systole (ms) | 11 | 300 ± 84 | 323 ± 38 | 0.42 |
| Diastolic filling time (ratio to cardiac cycle) | 11 | 0.49 ± 0.05 | 0.54 ± 0.05 | 0.059 |
| A wave duration (ms) | 11 | 140 ± 31 | 142 ± 31 | 0.90 |
| Left atrial ejection fraction | 11 | 0.42 ± 0.12 | 0.40 ± 0.10 | 0.57 |
| Longitudinal left ventricular systolic function | ||||
| Mean peak annular systolic velocity, s′ (cm/s) | 11 | 6.2 ± 1.5 | 6.0 ± 1.6 | 0.83 |
| Mean peak systolic displacement of the mitral annulus, D (mm) | 11 | 6.9 ± 1.3 | 7.4 ± 1.6 | 0.43 |
| Mean basal strain (6 segments) (%) | 11 | −12.7 ± 7.9 | −17.8 ± 7.8 | 0.22 |
| Mean basal strain rate (6 segments) (1/s) | 11 | −1.1 ± 0.7 | −1.4 ± 0.6 | 0.14 |
| Mean strain (12 segments) (%) | 11 | −14.3 ± 4.7 | −16.5 ± 4.4 | 0.29 |
| Mean strain rate (12 segments) (1/s) | 11 | −1.2 ± 0.3 | −1.2 ± 0.4 | 0.78 |
| Dyssynchrony indexes | ||||
| Maximal difference between time to peak annular systolic velocity (ms) | 11 | 67.7 ± 45.0 | 114.7 ± 40.2 | 0.015 |
| Maximal difference between time to peak systolic displacement (ms) | 11 | 47.3 ± 45.0 | 24.4 ± 23.6 | 0.16 |
| Yu index (ms) | 11 | 37.0 ± 19.3 | 52.7 ± 21.5 | 0.09 |
| Aortic preejection time (ms) | 11 | 75 ± 19 | 71 ± 16 | 0.56 |
| Aortic–pulmonary delay (ms) | 11 | 16.9 ± 37.6 | 10.3 ± 31.0 | 0.66 |
| Myocardial ischemia | ||||
| Wall motion score index | 11 | 1.24 ± 0.35 | 1.19 ± 0.30 | 0.72 |
| Number of segments with postsystolic shortening (16-segment model) | 11 | 3.7 ± 4.2 | 6.9 ± 7.8 | 0.25 |
| Mitral regurgitation | ||||
| Mitral regurgitation jet area (cm 2 ) | 3 | 4.0 ± 2.8 | 4.5 ± 2.2 | 0.82 |
| Mitral regurgitation jet area/left atrial area ratio | 3 | 0.13 ± 0.09 | 0.15 ± 0.08 | 0.85 |
| Tenting area (cm 2 ) | 11 | 2.1 ± 0.6 | 2.2 ± 0.7 | 0.75 |
| Mitral annular diameter (mm) | 11 | 28.9 ± 4.2 | 29.9 ± 3.5 | 0.56 |
| Right ventricular function | ||||
| Tricuspid annular peak systolic excursion (mm) | 10 | 20.7 ± 2.8 | 21.5 ± 2.7 | 0.51 |
| Peak annular systolic velocity (cm/s) | 10 | 15.7 ± 4.6 | 14.6 ± 4.4 | 0.61 |
| Right ventricular isovolumic acceleration (m/s 2 ) | 8 | 3.1 ± 1.5 | 2.6 ± 1.8 | 0.54 |
| Basal strain (%) | 7 | −28.5 ± 8.8 | −28.8 ± 6.9 | 0.95 |
| Basal strain rate (1/s) | 7 | −2.6 ± 0.6 | −2.1 ± 0.8 | 0.20 |
| Transtricuspid gradient (mm Hg) | 10 | 19.3 ± 10.6 | 17.7 ± 8.5 | 0.70 |
| Pulmonary vascular resistance (dyne×s×cm 5 ) | 10 | 118.9 ± 36.8 | 105.3 ± 21.7 | 0.37 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


