Bronchiolitis
DISEASES OF THE BRONCHIOLES
Diseases of the bronchioles occur throughout the bronchiolar structures, from bronchiolar airways to alveolar ducts and alveoli (Table 51-1). Acute and chronic bronchiolitis are seen from near the bronchi, all the way to the respiratory bronchioles; constrictive bronchiolitis is seen in the midbronchioles, while diffuse panbronchiolitis occurs from the distal bronchioles to the respiratory bronchioles, and smoker’s bronchiolitis involves the respiratory bronchioles. Bronchiolitis obliterans organizing pneumonia (BOOP) includes both the terminal bronchioles and alveoli and is discussed in Chapter 57.
New bronchiolar disorders continue to be described, including diffuse panbronchiolitis and smoker’s bronchiolitis. New causes of bronchiolitis obliterans have also been described, including lung transplantation, pulmonary microcarcinoids, Sauropus androgynous vegetable drink, and food-flavoring. This chapter includes a discussion of the pathological, clinical, radiographic findings, and treatment of the bronchiolar airway disorders.
BRONCHIOLAR ANATOMY
Bronchioles are noncartilagenous small airways which are usually 1 mm or less in diameter; they have been called the bridge between the bronchi and alveoli.1 The bronchioles have cartilage and mucus glands that are commonly found in the bronchi, but bronchioles also contain ciliated epithelium, smooth muscle, and Clara cells.2 Clara cells are columnar cells with apical surfaces capable of secreting proteins and surfactant. Neuroendocrine cells are common in the proximal bronchioles.
More distal in the airways are approximately 30,000 terminal bronchioles that have an average diameter of about 0.6 mm. These bronchioles have circular smooth muscles in the their walls; the surface cilia gradually disappear distally. Terminal bronchioles branch into 224,000 respiratory bronchioles that differ from the bronchioles: respiratory bronchioles have two to three alveolar structures in the walls containing columnar cells with cuboidal type II cells and squamous type I cells. These structures terminate in 13.8 million alveolar ducts and 300 million alveoli.
THE CLINICAL SPECTRUM OF THE BRONCHIOLAR DISEASES
The wide variety of bronchiolar diseases that may be seen in clinical practice are discussed below.
 ACUTE AND CHRONIC CELLULAR BRONCHIOLITIS
ACUTE AND CHRONIC CELLULAR BRONCHIOLITIS
Acute and chronic cellular bronchiolitis is characterized pathologically as acute or chronic inflammation of the bronchioles without a fibrotic component.3 The inflammation may be submucosal, mural, or peribronchiolar. Clinically, this is a common respiratory illness in children that is caused by several infectious agents, including Mycoplasma, adenovirus, influenza, parainfluenza, herpes virus, and adenoviruses. In the adult, bronchiolitis is rare and caused by similar viruses. Symptoms include a flu-like illness with persistent nonproductive cough of several weeks duration. There is generally no wheezing and no airflow obstruction. The chest x-ray is normal. The illness usually subsides over time. Cough suppressants may be utilized. Sometimes, a brief course of corticosteroid therapy is given for a severe, relentless cough. If symptoms are not responsive to corticosteroid therapy or if symptoms worsen, the illness may be fibrotic constrictive bronchiolitis – a disorder with a different clinical course and prognosis.
 RESPIRATORY BRONCHIOLITIS
RESPIRATORY BRONCHIOLITIS
Respiratory bronchiolitis is sometimes called smoker’s bronchiolitis, as cigarette smoking is almost always the cause of this lesion.3 The characteristic histological feature is the accumulation of tan-brown macrophages in the lumens of respiratory bronchioles and adjacent alveoli-often seen as an incidental finding in cigarette smokers. For example, respiratory bronchiolitis was found in 70 of 79 (88.6%) smokers who underwent surgery for spontaneous pneumothorax.4 There are usually no clinical symptoms associated with this type of respiratory bronchiolitis. Treatment is smoking cessation.
In some situations, respiratory bronchiolitis may extend into the interstitium and is referred to as respiratory bronchiolitis-interstitial lung disease (RB-ILD). Affected individuals have shortness of breath, bilateral crackles, reticulonodular opacities on chest radiography, decreased vital capacity, and decreased diffusing capacity. Treatment is smoking cessation, but often a course of corticosteroid therapy is needed for resolution.
 FOLLICULAR BRONCHIOLITIS
FOLLICULAR BRONCHIOLITIS
Follicular bronchiolitis is characterized by hyperplastic lymphoid aggregates forming 1- to 2-mm peribronchiolar nodules.3 Follicular bronchiolitis is often limited to a pathological description with no clinical counterpart. It occurs in the connective tissue disorders, such as rheumatoid arthritis. In some patients, there may be cough, sputum production, and small linear radiographic opacities.5
 DIFFUSE PANBRONCHIOLITIS
DIFFUSE PANBRONCHIOLITIS
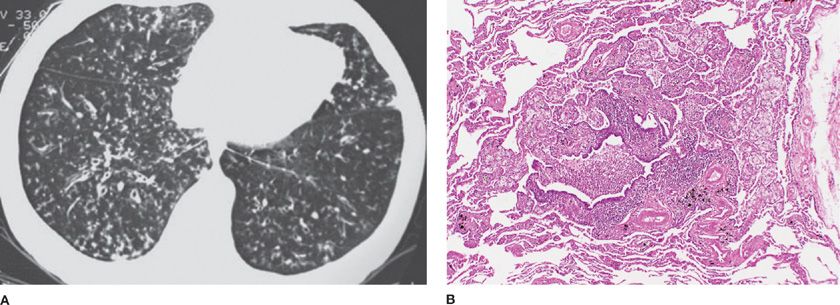
Diffuse panbronchiolitis was first described in the 1960s and is characterized by chronic inflammation and lymphofollicles of the respiratory bronchioles and adjacent centrilobular regions, with infiltration of histiocytosis, plasma cells, and lymphocytes.6 There is also an interstitial accumulation of foam cells in the walls of respiratory bronchioles and adjacent alveolar ducts and alveoli (Fig. 51-1). The disorder is largely restricted to the Asian countries, but diffuse panbronchiolitis has been reported in the United States, Australia, Canada, and Spain.7–10 The disorder has been reported in Kartagener syndrome.11 In recent years, there appears to be a major decrease in the incidence and prevalence of diffuse panbronchiolitis in Japan.12
Figure 51-1 Diffuse panbronchiolitis. A. The chest CT scan shows bilateral centrilobular nodules with branching showing “tree-in-bud” pattern. B. The pathology micrograph shows chronic inflammation of the respiratory bronchioles, with interstitial accumulation of foam cells in the walls of the respiratory bronchioles, adjacent alveolar ducts, and alveoli. (Used with permission of Dr. Kenneth W. Tsang, Queen Mary Hospital, Hong Kong, China; and Dr. Thomas V. Colby, Lung Pathology, Mayo Clinic Scottsdale.)
Symptoms include chronic cough, sputum production, shortness of breath, and almost all individuals have chronic paranasal sinusitis. Rhonchi and crackles are common. Radiographic findings show hyperinflation and diffuse small nodular opacities bilaterally. Pulmonary function testing shows airflow obstruction with decreased forced expired volume in one second (FEV1) and FEV1 to forced vital capacity (FEV1/FVC) ratio. Hypercapnia and cor pulmonale occur late. There is often an associated increase in the cold-hemagglutinin titer. There appears to be a major susceptibility gene located between the HLA-A and HLA-B loci on the short arm of chromosome six.13
The disorder is progressive. In the past, diffuse panbronchiolitis was a fatal disease with a 10-year survival of less than 20% in patients with Pseudomonas aeruginosa infection. However, the introduction of low-dose, long-term erythromycin and other macrolides has resulted in a dramatic improvement in survival. Among Chinese patients, Li et al.14 showed azithromycin, 500 mg once daily, for 3 months and 500 mg three times weekly for 6 to 12 months, resulted in complete cure in 27.5% of patients, elimination of symptoms in 70.6%, and a 5-year survival of 94.1%. The macrolides may prevent influx of neutrophils into the alveoli, decrease interleukin-8, and interfere with the pathological potency of P. aeruginosa.15
 BRONCHIOLITIS OBLITERANS
BRONCHIOLITIS OBLITERANS
Bronchiolitis obliterans is an important lesion because it can be severely disabling and deadly. Traditionally, bronchiolitis obliterans has been used as a clinical term to describe irreversible fibrosis of the bronchiolar airway that is idiopathic or occurs after accidental toxic fume inhalation or a viral pneumonia. However, pathologists may see two distinctive lesions that, in turn, have a different clinical course and response to treatment.
Histologically, the two lesions are proliferative bronchiolitis and constrictive bronchiolitis (Table 51-2). Depending on severity, obliteration of the bronchioles, the obliterans term, may or may not occur with these lesions. The histological distinction between these two lesions is that constrictive bronchiolitis arises in a concentric fashion outside the bronchiole walls as a fibrotic lesion, and proliferative bronchiolitis arises from within the bronchiole walls as an inflammatory lesion.
The term bronchiolitis obliterans is used in this chapter, as it has been used by clinicians for more than a hundred years and almost always reflects the fibrosing, constrictive, pathological lesion.16 The proliferative lesion is often self-limiting and less severe, or responds to corticosteroid therapy with complete resolution; it usually does not result in the clinical label of bronchiolitis obliterans.
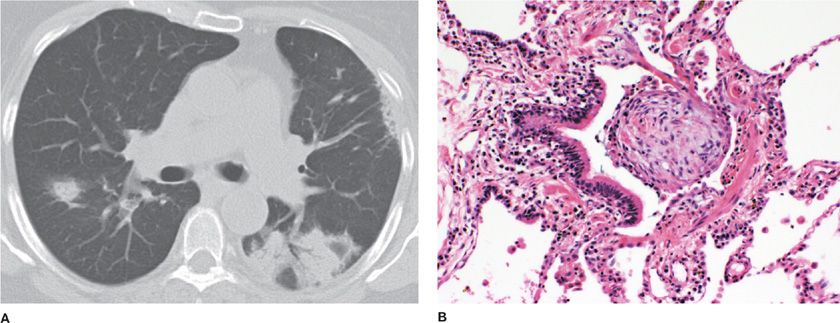
Proliferative bronchiolitis is an inflammatory bronchiolitis characterized by intraluminal polypoid connective tissue masses of myxoid fibroblastic tissue which resembles granulation tissue that arises from within the bronchioles.1 Central clusters of mononuclear inflammatory cells may be found in these polypoid masses. This type of bronchiolitis includes organized polypoid granulation inflammatory tissue in the distal bronchiole airways, respiratory bronchioles, alveolar ducts, and alveoli in the form of BOOP17 Additional distinctive histological findings with proliferative bronchiolitis associated with BOOP include no disruption in the lung architecture, interstitial fibrosis, absence of traction bronchiectasis, or histological honeycombing (Fig. 51-2).
Figure 51-2 Bronchiolitis obliterans organizing pneumonia. A. The chest CT scan shows bilateral patchy ground-glass opacities, air bronchograms, and peripheral-based triangular infiltrates. B. The pathology micrograph shows organized polypoid granulation tissue filling the distal bronchiole and extending into the alveoli. (Used with permission of Dr. Ritu R. Gill, Chest Radiology, Brigham and Women’s Hospital, Boston, and Dr. Thomas V. Colby, Lung Pathology, Mayo Clinic Scottsdale.)
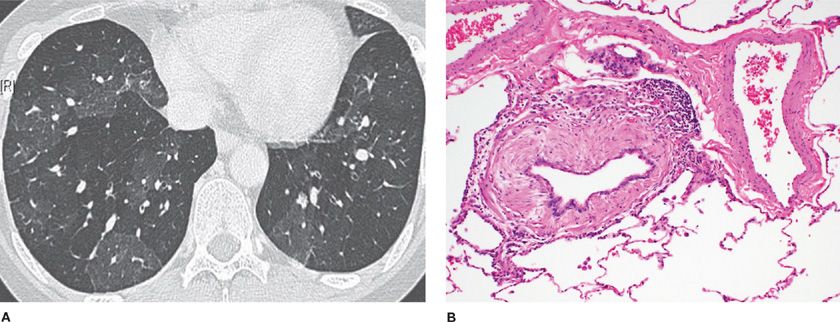
Constrictive bronchiolitis is a fibrotic, concentric bronchiolitis lesion with or without complete obliteration (Fig. 51-3). This lesion is usually seen in the mid to distal area of bronchioles and does not extend into the respiratory bronchioles or alveoli. The lesion is characterized by a peribronchiolar fibrotic process that surrounds, rather than fills, the lumen, resulting in extrinsic compression and obliteration of the airway.3 There is mural thickening by submucosal collagenous fibrosis with progressive concentric narrowing associated with luminal distortion, mucus stasis, and chronic inflammation.18
Figure 51-3 Constrictive bronchiolitis. A. The expiratory chest CT scan shows the mosaic pattern of air trapping seen from obliterated bronchioles. B. The pathology micrograph shows extrinsic fibrosis of the bronchiolar wall in a concentric manner constricting and obliterating the bronchiolar lumen. (Used with permission of Dr. Ritu R. Gill, Chest Radiology, Brigham and Women’s Hospital, Boston, and Dr. Thomas V. Colby, Lung Pathology, Mayo Clinic Scottsdale.)
Constrictive bronchiolitis was called “fibrosing bronchiolitis” in the German pathology literature.19 The lesion preferentially involves membranous bronchioles and is characterized by fibrosis of the stroma and narrowing the lumen in a concentric fashion. The muscle layer may be hypertrophic in early lesions, atrophic in late stages, and replaced by fibrotic tissue at the end stage. Visscher and Myers noted that constrictive bronchiolitis is often patchy and focal, making the diagnosis difficult from a transbronchial biopsy; advanced cases may be especially inconspicuous because of lack of active inflammation and disappearance of bronchioles.20
There are several causes of bronchiolitis obliterans and associated systemic disorders (Table 51-3).
Idiopathic Bronchiolitis Obliterans
Idiopathic bronchiolitis obliterans occurs among individuals who have no obvious inciting agent or associated systemic disorder, and who have airflow obstruction and constrictive bronchiolitis histologically. This disorder continues to be exceedingly rare. Symptoms begin with a nonproductive cough, and shortness of breath develops later. Physical examination shows no wheezing but may demonstrate an unusual finding of early inspiratory crackles. The crackles occur early because of the scarring around the midbronchiole airways, causing snapping closure of the airways and resultant crackling sound.
Pulmonary function studies show irreversible decrease in the FEV1 and FEV1/FVC with no improvement after bronchodilator inhalation. The diffusing capacity is highly variable, from increased, to normal, to markedly decreased.
The chest roentgenogram is often normal or shows hyperinflation. High-resolution chest CT scans during inspiration and expiration can be helpful for establishing a diagnosis (Fig. 51-1). For example, a 65-year-old woman with idiopathic bronchiolitis obliterans had a normal chest CT scan in the inspiratory study, but the expiratory study showed extensive lobular air trapping.21 These expiratory images show low-attenuation areas in the secondary pulmonary lobules, resulting in a typical mosaic pattern with scattered areas of low attenuation. Additional thin-section CT findings include constriction of the pulmonary vessels within the low-attenuation areas, expiratory air trapping, bronchial dilation, and sometimes, centrilobular nodules or branching linear densities.22
Treatment consists of high-dose corticosteroids such as prednisone with an initial dose of 60 mg daily, followed by lower-dose therapy for usually one year in patients who respond. For those who do not respond by three months, corticosteroids are discontinued and employed for life-threatening exacerbations. Immune-suppression treatment can be utilized and lung transplantation for individuals with life-threatening and severe disease.
Patients who survive the initial episode may stabilize for several years or progress to end-stage airflow disease and cor pulmonale.23 For example, a 43-year-old woman had idiopathic bronchiolitis obliterans for 24 years and showed relentless progression of airway obstruction with 19 admissions for respiratory failure.24 The autopsy in this patient showed complete obliteration of smaller airways. The FEV1 decreased from 1.06 L to 0.40 L terminally.
Myong et al.25 described three women aged 41 to 54 who developed cough and progressive shortness of breath during 6 months to 10 years; lung tissue showed constrictive bronchiolitis with bronchiolar airway obliteration. The thin-section CT scans showed low-attenuation changes of the mosaic pattern. None responded to corticosteroid treatment. One woman died 8 months after the diagnosis from lymphoma and the other two were stable.
Toxic Fume Bronchiolitis Obliterans
Toxic fume bronchiolitis obliterans is a three-phase response disease. The exposure usually occurs from an accidental explosion resulting in nose, throat, and eye irritation with no major respiratory symptoms. Phase one is an asymptomatic latency period of 6 to 12 hours after exposure. Phase two begins suddenly with acute-onset respiratory failure and acute respiratory distress syndrome. Successful treatment results in another asymptomatic latency period of 7 to 10 days. Phase three then occurs as constrictive bronchiolitis with irreversible airflow obstruction, progressive shortness of breath, and chronic respiratory failure.
This disorder occurs after accidental exposures to sulfur dioxide fumes, nitric acid fumes, and nitrogen dioxide in freshly filled corn silos. Unusual accidental exposures to toxic fumes may cause this lesion. For example, two workers in a lithium battery factory were accidentally exposed to thionyl chloride, and one of them developed findings consistent with bronchiolitis obliterans.26 This acidic compound is used in the manufacturing process and produces sulfur dioxide and hydrochloric acid fumes when in contact with water.
Smoke inhalation bronchiolitis obliterans was described in a 23-year-old man who was in a fire while sleeping in his newly constructed house.27 He was unconscious when rescued. There was cough and mild dyspnea after recovery. He returned 3 years later because of persistent dyspnea. He had finger clubbing, an FEV1 of 0.90 L, and an FEV1/FVC of 34%. The burning synthetic structural materials used to build his house produced gases containing acrolein, formaldehyde, acetaldehyde, nitrogen dioxide, and sulfur dioxide.
There has been a report of bronchiolitis obliterans from mustard gas occurring from a chemical warfare attack in a 37-year-old man who had cough, sputum production, shortness of breath, and airflow obstruction for 14 years after the exposure.28 Later, investigators29 used high-resolution chest CT scan findings for the diagnosis of bronchiolitis obliterans in a group of individuals exposed to the same mustard gas attack. They treated 18 individuals with bronchodilator treatment and 18 with interferon gamma-1b and 7.5 mg of prednisolone. Patients had baseline FEV1 values of 49.3% and 48.7% predicted, respectively. Both groups improved after 6 months of treatment; however, the group treated with interferon gamma had a significantly higher posttreatment FEV1 of 66.3% compared to 57.3% for the group treated with bronchodilators (p = 0.001).
King et al.30 reported constrictive bronchiolitis among 38 US soldiers returning from Iraq and Afghanistan and found a common exposure to a sulfur-mine fire in 2003 among 28 of them.
A 42-year-old police officer exposed to the dust in the cloud from the New York City World Trade Center disaster of September 11, 2001 developed decreased FEV1 and FEV1/FVC in April 2002.31 The lung biopsy showed regions of constrictive bronchiolitis. He was treated with oral corticosteroid therapy and azithromycin. By April 2003, pulmonary function studies had returned to normal values.
An unusual radiographic appearance of scattered lung cysts has been reported in a 49-year-old woman with severe airflow obstruction and constrictive bronchiolitis.32
Diacetyl appears to be a common exposure among artificial butter flavoring workers who developed constrictive bronchiolitis. A report of nine microwave popcorn factory workers showed airflow obstruction among the mixers, and biopsy of some individuals showed constrictive bronchiolitis.33 Investigators34 have reported that four workers at a cookie factory who were exposed to diacetyl developed severe and persistent airflow obstruction, with FEV1 ranging from 25% to 44%. Lung biopsy showed constrictive bronchiolitis and bronchiolar airway distortion.
Post-Respiratory Infection Bronchiolitis Obliterans
Post-respiratory infection bronchiolitis obliterans may occur after adenovirus pneumonia, influenza or parainfluenza pneumonia, or after Mycoplasma pneumonia.35 Cough develops several days after the initial infection. Chest radiographs may show diffuse reticulonodular opacities early but are normal or show hyperinflation late. Expiratory, high-resolution chest CT scans show low-attenuation mosaic pattern. Tissue shows constrictive bronchiolitis with extensive scarring that obliterates many of the bronchioles, corresponding clinically with severe airflow obstruction. At this stage, the lesion is not responsive to corticosteroid medication. Lung transplantation has been utilized for severe post-Mycoplasma pneumonia bronchiolitis obliterans.36
Connective Tissue Bronchiolitis Obliterans
Connective tissue bronchiolitis obliterans occurs most commonly in rheumatoid arthritis and has been reported in scleroderma, lupus erythematosus, and Sjögren’s syndrome.37,38
Rheumatoid arthritis-related constrictive bronchiolitis often has a poor prognosis. Among 25 individuals with rheumatoid arthritis and bronchiolitis obliterans, most had severe airflow obstruction, often with an FEV1 less than 1 L, and the process was not responsive to corticosteroids.39 The outcome was poor, as chronic respiratory failure occurred in 40% of the patients; four patients died.
Drug-Related Bronchiolitis Obliterans
Drug-related bronchiolitis obliterans has been reported with penicillamine and gold used for treatment of rheumatoid arthritis. The penicillamine-related bronchiolitis obliterans has a poor prognosis, sometimes requiring lung transplantation for management.40 Fatal bronchiolitis obliterans has been reported in a 12-year-old girl with juvenile rheumatoid arthritis after a 6-month course of intramuscular gold.41 Although cause and effect are difficult to confirm for both of these agents, patients receiving these medications who develop unexplained cough or dyspnea need to be evaluated for the possibility of bronchiolitis obliterans.
Bone-Marrow Transplantation Bronchiolitis Obliterans
Bone-marrow transplantation bronchiolitis obliterans occurs much less frequently as allogeneic stem-cell transplantation has become so common; however, bronchiolitis obliterans may occur in up to 9% of allogeneic bone-marrow recipients.42 Bronchiolitis obliterans occurs only after graft-versus-host reaction, and therefore, is rarely seen after autologous bone-marrow transplantation. As complications of chronic graft-versus-host develop after 100 days, bronchiolitis obliterans is usually seen 6 to 12 months after transplantation. Donor type-2 T-helper lymphocytes appear to be the primary mediators. The pathological lesion is concentric bronchiolar fibrosis typical of constrictive bronchiolitis. There is generally a poor response to corticosteroid therapy; mortality ranges from 40% to 100%.42 Living donor lobar lung transplantation has been used successfully for bone-marrow transplant-related bronchiolitis obliterans.43
Stem-Cell Transplant Bronchiolitis Obliterans
Stem-cell transplant bronchiolitis obliterans has replaced bone-marrow transplantation-associated bronchiolitis obliterans as allogeneic hematopoietic stem-cell transplantation has become more widespread (see Chapter 95). The prevalence of bronchiolitis obliterans ranges from 2% to 3% among all allogeneic recipients to 6% among those who develop chronic graft-versus-host disease (cGVHD).44 These data underestimate the incidence of bronchiolitis obliterans (26%) when using an annualized rate of decline in FEV1 of 5%. Those with cGVHD had a rate of 30%, but more importantly, they had a mortality rate of 40% at 10 years. cGVHD is the major risk factor for bronchiolitis obliterans, as high as 80% of patients with bronchiolitis obliterans are preceded with cGVHD. Other risk factors include low IgG levels, use of peripheral blood stem cells, poor pretransplant lung function, and respiratory infection during the first 100 days. Biopsy specimens performed early show bronchiolitis, fibrous obliteration of the respiratory bronchioles, and inflammatory cell infiltrates; later lesions show constrictive bronchiolitis with circumferential fibrosis. The mechanism of bronchiolitis obliterans is based on chronic rejection that may involve donor cytotoxic T cells.
Preventive treatment of stem-cell transplantation bronchiolitis obliterans consists of early and aggressive treatment of respiratory infections and cGVHD. Treatment includes high-dose systemic corticosteroids and immunosuppression with calcineurin inhibitors, sirolimus, azathioprine, and anti-thymocyte globulin. Prognosis continues to be poor despite treatment and supportive care, with an overall survival rate of 44% at 2 years and 13% at 5 years.44
In a study of 2087 allogeneic stem-cell transplantation recipients from 1994 to 2005, there were 57 (2.8%) who developed bronchiolitis obliterans.45 The time interval between transplantation and bronchiolitis obliterans ranged from 83 days to 907 days with a median time of 335 days. Acute graft-versus-host disease was not found to be a risk factor, whereas cGVHD was a significant risk factor. The development of bronchiolitis obliterans was related to the stem-cell source, with related peripheral blood stem-cell transplantation (3.83%) the highest. Unrelated bone-marrow transplantation (2.91%) and cord-blood transplantation (2.65%) were lower, with related bone-marrow transplantation (1.62%) the lowest. The outcome among these 57 patients showed that 8 (16.7%) improved, 10 (21.7%) showed no change, and 28 (60.9%) died; the cause of death was respiratory failure in 17 (60.7%).
The high-resolution chest CT findings in bronchiolitis obliterans among patients receiving allogeneic stem-cell transplantation shows geographic hypoattenuation and air trapping with subpleural predominance.46 In some patients, the geographic hypoattenuation involved more than half of both lungs.
Extracorporeal photodynamic therapy has been used for the treatment of cGVHD and bronchiolitis obliterans.47 The role of this treatment in the management of bronchiolitis obliterans in stem-cell transplantation recipients has not yet been established. Some patients have improved pulmonary function.
Mesenchymal stem-cell treatment was used for one patient. A 38-year-old patient received peripheral blood stem cells from her sibling and developed bronchiolitis obliterans 8 months later with an FEV1 <0.7 L.48 She was treated experimentally with human bone marrow–derived mesenchymal stem cells from her sister on day 275 and on day 305, resulting in disappearance of symptoms and improved pulmonary function. This may be an effective treatment on a case-by-case basis, but at this time, there are too many unknown variables involved to advocate its use (e.g., absence of large scale studies, availability of a standardized source of mesenchymal cells, and an understanding of potential carcinogenic effects).
Lung Transplant Bronchiolitis Obliterans
Lung transplant bronchiolitis obliterans has emerged as the most important clinical complication among lung transplant recipients since the mid-1980s and has continued to plague thoracic surgeons and patients, with minimal change in occurrence and mortality (see Chapter 107).
The terminology has become the bronchiolitis obliterans syndrome (BOS), which is a clinical classification based on FEV1. The classification was developed because BOS is a common problem among lung transplantation recipients. The approach eliminates the need for low-yield transbronchial biopsy or other invasive procedure to establish a definitive diagnosis of bronchiolitis obliterans. A clinical severity classification has been used.49 A National Institutes of Health (NIH) diagnostic classification of BOS in cGVHD50 has also been developed (Table 51-4).