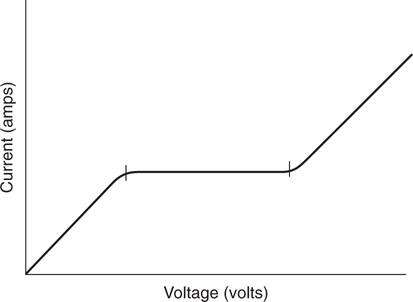
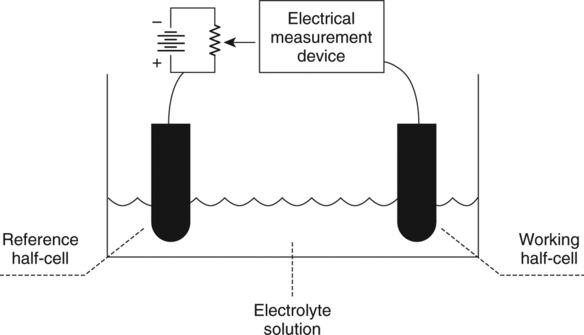
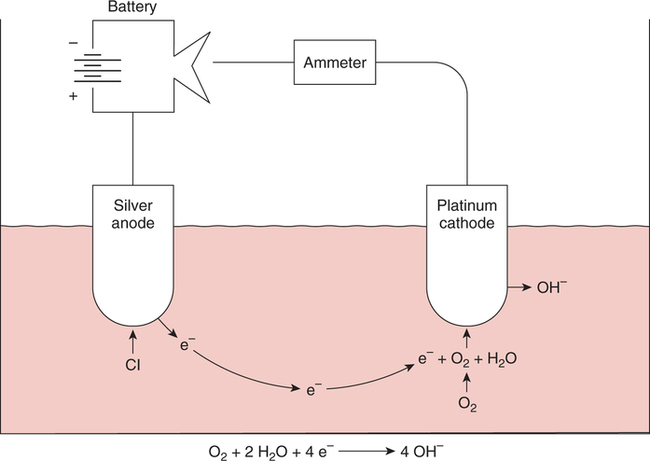
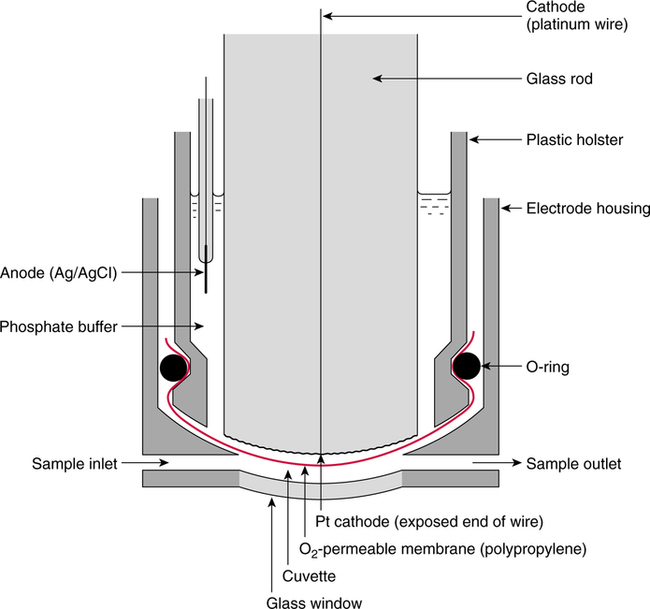
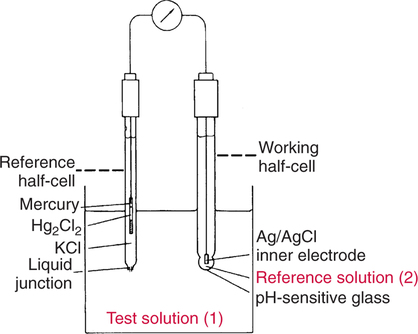
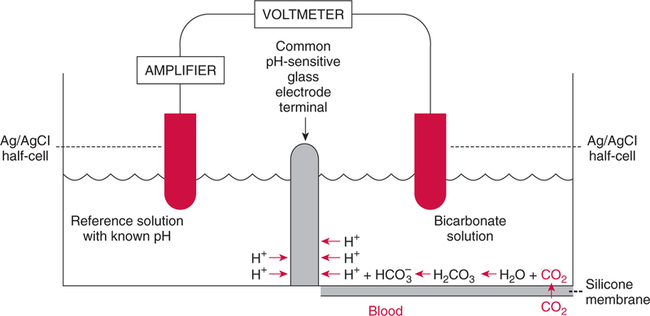
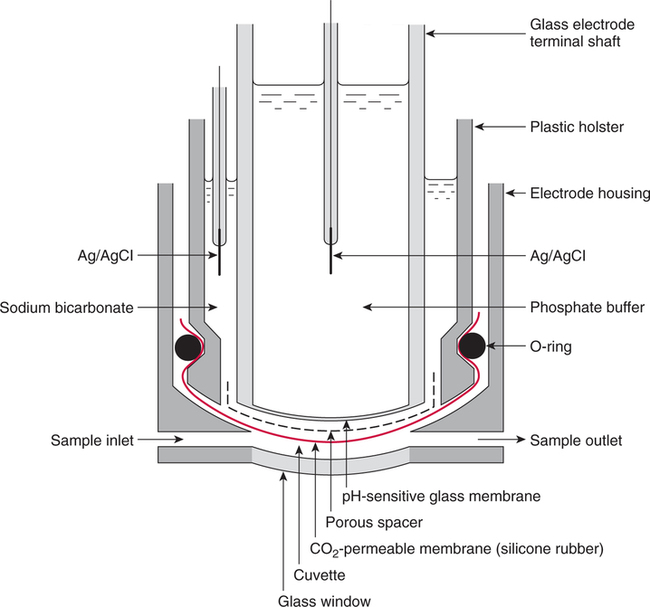
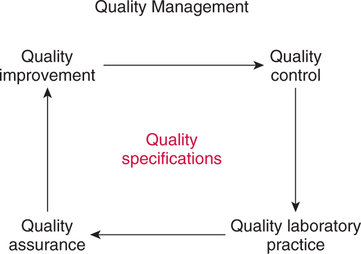
The force responsible for pumping theseelectrons is called the electromotive force or potential.120 The greater the difference in electron concentration between the two poles, the greater is the electromotive force. The unit of measurement for electromotive force is the volt. Voltage refers to electromotive potential, and actual electron flow does not occur unless a conductor bridges the positive and negative poles. A potentiometer is an instrument that measures an unknown voltage by comparing it with a known reference voltage.122 In chemistry, an electrode is defined technically as an electric conductor or terminal through which electricity enters or leaves a medium such as an electrolyte solution.122 By usingthis terminology, an electrochemical cell isan apparatus that consists of two electrodes placed in an electrolyte solution.121 122 Similarly, an electrochemical cell system is an apparatus that incorporates one or more electrochemical cells to measure a specific chemical species. Thus, from a chemical standpoint, all the traditional analytical devices used in blood gas analysis should be referred to as electrochemical cell systems. Similarly, within each measuring device there would be at least two electrodes. Nevertheless, clinically, these entire electrochemical cell systems are referred to almost invariably as simply “blood gas electrodes.”123 Therefore, this clinical terminology is used in the text in an effort to promote consistency and avoid confusion in a commonly misunderstood subject. The entire measurement device is referred to as an electrode (e.g., PO2 electrode, pH electrode, PCO2 electrode), and the term electrode is reserved solely for this use. There are two types of half-cells: working half-cells and reference half-cells. The working or measuring half-cell is placed at the site where the actual chemical analysis, work, or electrochemical change takes place.121 123 The reference half-cell is the standard against which the electrochemical change is compared and measured (Fig. 4-1). A reference half-cell typically consists of a solid metal and a solution of its salt (e.g., silver and silver chloride, mercury and mercurous chloride) attached to an electronic circuit. When the metal is in contact with its salt solution, a constant electrical potential or voltage is produced.121 The PO2 electrode incorporates a battery and an ammeter as the electrical components of the electrode (Fig. 4-2).123 Wall electricity may be used in place of a battery. The electrode terminal in the working half-cell is usually made of platinum. The electrode terminal in the reference half-cell is made of silver/silver chloride. The platinum is negatively charged and serves as a cathode in the electrical system, whereas the silver is positively charged and serves as the anode. To initiate the flow of electrical current and the measurement of oxygen, the battery supplies the platinum cathode with a voltage of approximately 700 millivolts (mV).124 This voltage attracts oxygen molecules to the cathode where they react with water. The ensuing chemical reaction consumes four electrons and produces some hydroxyl ions (see Fig. 4-2). The consumed electrons, in turn, are replaced rapidly in the electrolyte solution as silver and chloride react at the anode. The net result of these reactions is a flowof electrical current throughout the entire circuit, which is shown in Figure 4-2. The current generated will be in direct proportion to the amount of dissolved oxygen (PO2) presentat the cathode (Fig. 4-3). An ammeter is a device used to measure the flow of electrical current. The direct relationship between PO2 and electrical current is true only when a specific voltage is applied initially to the cathode.123 The proper voltage to use is determined by analyzing a polarogram, which is a graph that shows the relationship between voltage and current at a constant PO2 (Fig. 4-4). The electrode must operate on the plateau of the polarogram to preserve the relationship between the PO2 and the current. Thus, PO2 analysis via the oxygen electrode is often referred to as a polarographic technique of gas analysis.123 The electrode shown in Figure 4-2 is not practical for the clinical measurement of blood PO2 because protein from the blood deposits on the cathode and alters its electrical characteristics. Clark introduced a clinical version of this electrode in 1953. An illustration of a modern Clark electrode is shown in Figure 4-5. In the Clark electrode, blood is separated from the electrode terminals by use of a special membrane, which is permeable to oxygen and is a good electrical insulator. Oxygen from the blood can diffuse easily through the membrane into the electrolyte solution in which the reaction with water can take place. The terminals in the electrode are not bathed directly in blood; they are bathed instead in a phosphate buffer solution, and potassium chloride is added.121 Most PO2 electrodes use polypropylene membranes; however, Mylar, Teflon, and polyethylene all have similar properties and may be used.123 The membrane is usually secured on the electrode with a rubber O-ring (see Fig. 4-5). In addition, the actual blood sample remains in a chamber known as a cuvette where it is warmed quickly to 37°C and is protected from contamination by the air. The pH electrode differs greatly from the PO2 electrode in both structure and function. Functionally, the pH electrode measures changes in voltage rather than actual electrical current. Specifically, pH is measured by the potentiometric method by which an unknown voltage is measured by comparison with a known voltage and is then converted to pH.123 To accomplish this, the pH electrode requires four electrode terminals instead of just two, such as in the case of the PO2 electrode. A reference solution of known pH is placed between two of the terminals to have reference voltage to compare the unknown voltage against. A single unique pH-sensitive glass electrode terminal serves as a common electrode terminal for both the reference solution and the solution of unknown pH (Fig. 4-6). The relationship between voltage and pH at a particular temperature is described by the modified Nernst equation.10 In general, for each pH unit difference between the known and unknown solutions, a difference of 61.5 mV develops. A special voltmeter converts voltage to pH units based on the Nernst equation and visually displays the pH. Actual electron flow (current) secondary to the voltage differences between the two sides is prevented by use of an amplifier between the voltmeter and the reference solution. This amplifier has high electrical resistance that prohibits significant electrical current and this allows for measurement of small voltage differences.125 The physical components of a model pH electrode are shown in Figure 4-7. This systemcan be divided physically into two major components. One component has the pH-sensitive glass at the tip and a silver/silver chloride half-cell inside it. Because this component is the site at which the actual blood comes in contact with the outer surface of the pH-sensitive glass, it may be referred to as the working half-cell. Thus, the mercury/mercurous chloride (Hg/Hg2Cl2) component can be called the reference half-cell. The reference half-cell shown in Figure 4-7 includes an electrode terminal made of mercury coated with mercurous chloride. The term calomel is often used for this type of electrode terminal. The calomel electrode terminal interfaces with a platinum wire that transmits the change in voltage to the voltmeter. Although blood could be sampled in a pH electrode similar to the one shown in Figure 4-7, this particular configuration presents several problems. This system would require a large blood sample volume. Furthermore, the blood would not be at body temperature and would be exposed to air. The PCO2 electrode (shown in Figure 4-8) is a modified version of the pH. In the PCO2 electrode, however, blood does not come in direct contact with the pH-sensitive glass. Rather, blood comes in contact with a CO2 permeable membrane. The membrane may be made of silicone rubber, Teflon, or a similar substance that is readily permeable to CO2. As shown in Figure 4-8, a chemical reaction occurs within the bicarbonate solution as CO2 diffuses in. This reaction, which is known as the hydrolysis reaction, results in the production of hydrogen ions and a pH change of the bicarbonate solution. The pH change is in direct proportion to the PCO2. Thus, the corresponding voltage change can be converted into PCO2 units and reflected on the voltmeter. A relatively high degree of precision can be expected in the analysis of arterial blood gases today.10 The approximate accuracy of blood gas electrodes is shown in Table 4-1. 10 123 126 127 128 129 Table 4-1 Accuracy of Blood Gas Electrodes The pH is repeatedly the most reliable and accurate measurement. The PCO2 variation in historical studies and reports was in the range of 1 to 5 mm Hg.123 126 127 128 129 130 Today, the varia-tion in PCO2 should be less than 1 mm Hg. Older PO2 electrodes were the least accu-rate with reports of as high as 20% inaccuracy, especially at high PO2 values.10 131 Variation in PO2 had been reported to be in the range of 3 to 20 mm Hg at normal PO2.126 127 128 129 These inaccuracies in the PO2 electrode were due at least in part to the production of hydrogen peroxide at the cathode site. Furthermore, gases such as carbon dioxide, halothane, and nitrous oxide may cause small changes in current in the system.123 Newer designs in PO2 electrodes, however, continue to make this electrode more accurate. Currently, PaO2 results should be expected to be accurate to nearly 3 mm Hg at 80 mm Hg.10 Current quality management in the laboratory is much more sophisticated and clinically oriented than in the past. Quality methods and techniques are focused on insuring that the quality of tests performed in the laboratory allow our clinicians to practice good medicine.362 We are becoming increasingly concerned with exactly how the laboratory results impact clinical decision-making. Are they being used for diagnosis, monitoring, or identifying critical levels? This is a much broader concept and goal than simply ensuring that test numbers fall within specific predefined limits. Thus, many issues and concerns that were previously ignored in laboratory quality management are now becoming increasingly important. Depending on the particular test, we may be very concerned with accuracy, turn-around time, cost, simplicity, sample size, etc. All these various attributes of a particular test are referred to as performance characteristics.362 Performance characteristics, in turn, can be subdivided into practicability characteristics (e.g., sample size, speed of analysis) and reliability characteristics (e.g., precision, bias). Ideally, quality specifications would be available for every performance characteristic.362 Quality specifications are the heart of a quality management system as shown in Figure 4-10. Note that in Figure 4-10, quality manage-ment encompasses quality assurance, quality improvement, and quality control. A detailed discussion of laboratory quality management is beyond the scope of this text. Excellent resources are available from the National Committee of Clinical Laboratory Standards [NCCLS], the American Association for Respiratory Care, and books362 related to this topic. Nevertheless, some of the routine components in quality management within the blood gas laboratory will be briefly discussed. Quality assurance is a systematic process used to monitor, document, and regulate the accuracy and reliability of a procedure or laboratory measurement. Errors in blood gases may occur before, during, or after actual analysis of the sample. An error that occurs before or after actual analysis (e.g., an error due to improper sample or data handling) is called a non-analytical error. On the other hand, an error that occurs during the actual analysis of the sample (e.g., error due to performance of electrode or technician) is called an analytical error.10 123 As described in Chapter 3, an error may be easily introduced into blood gas data during arterial puncture or sample handling. The sample may be inappropriately drawn or transported. Blood gas results could be attributed inadvertently to the wrong patient. A patient’s status or therapy may be incorrectly assessed or recorded. Preanalytical error is likely to be the greatest source of incorrect blood gas data. If left unnoticed, this error may have serious consequences on the treatment of the patient. Recording of results after analysis may be associated with an error in transcription. In particular, the use of telephone reports may easily lead to serious reporting error due to a breakdown in verbal communication.132 Telephone reporting is done typically in the critical care setting and in regard to critically ill individuals. These individuals cannot afford the potential consequences of incorrect information. The incidence of this type of error may be reduced if the individuals who receive these data are knowledgeable of normal clinical ranges for blood gases.132 It is also very important that the individuals receiving the data read it back to confirm accuracy. Finally, knowledge of potentially life-threatening values for these parameters also helps to prevent a serious error being made. Ideally, results are directly printed out from the machine at a remote location, and the potential for human error is eliminated. In summary, blood gas results must be interpreted in light of the potential for both preanalytical or postanalytical error. Unexpected blood gas results should arouse suspicion. Blood gases should be repeated immediately, preferably on another instrument if: (1) they are inconsistent with the patient picture, (2) they are internally inconsistent (see Chapter 5), (3) they are at the extremes of the expected range.241 An example of human analytical error, however, is failure on the part of the technician to properly mix the sample before it is introduced into the electrode. Failure to mix an iced sample may increase the pH of the sample by as much as 0.11 units.123 Also, the technician should not record blood gas values immediately after injecting the sample into the electrode. An adequate exposure time is necessary to achieve sample stabilization at body temperature, ambient pressure, saturated, and to ensure complete electrode response. Most samples achieve equilibrium within 1 to 3 minutes. Two or three times longer may be necessary, however, if PCO2 is extremely low or if PO2 is extremely high in the sample.125 133 Another source of error is inherent biologic variation.362 The difference between individuals is called between-subject or inter-individual biologic variation. There are two general types of biologic variation. The difference in a given individual is called within-subject or intra-individual variation. Intra-individual variation may occur during daily, monthly, or yearly cycles or during the aging process. Calibration is a procedure done on blood gas electrodes before analyzing blood samples to establish the accuracy of readings in the anticipated range. Standards are gases or buffer solutions with precise, specific blood gas values that are used to set the machine to read linearly over the physiologic range. Gases used forcalibration should be extremely accurate and should be traceable to National Institute of Standards and Technology (NIST) certification. Due to the many different protocols, designs, and recommendations of various manufacturers, no specific guidelines can be provided for routine calibration. Operators must adhere to specific manufacturer’s recommendations.241 An additional problem with the PO2 electrode is that the PO2 reading is lower if a gas is introduced into the electrode than if a liquid is introduced into the electrode. This discrepancy has been referred to as the fluid-gasdifference,123 the blood-gas factor,10 123 or the stirring effect.125 A rough correction factor for the fluid-gas difference is 1.04 × PO2 of the gas sample.123 The mean is a fundamental statistic that is calculated by dividing the sum of all the numbers in a group by the number of numeric entries. In lay terms, the mean is known as the average. Mathematical calculation of the mean is shown in Equation 4-1. The normal range is shown well by the bell-shaped curve described in Chapter 1 (see Fig. 1-1). The degree of dispersion (i.e., scattering of values from the average) in a group of numbers can be quantitated by calculating the SD. The SD is therefore a measure of variance around the mean. The formula for calculation of the SD is shown in Equation 4-2.
Blood Gas Electrodes and Quality Assurance
BLOOD GAS ELECTRODES
Basic Electrical Principles
Voltage
Terminology
Electrochemical Cell Systems
Half-Cells
Structure and Function
PO2 Electrode
Basic Components
Electrochemical Reaction
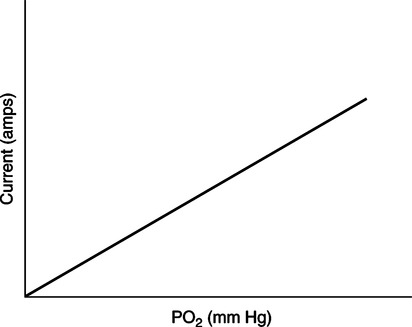
Polarography
Clark Electrode
pH Electrode
Electrode Function
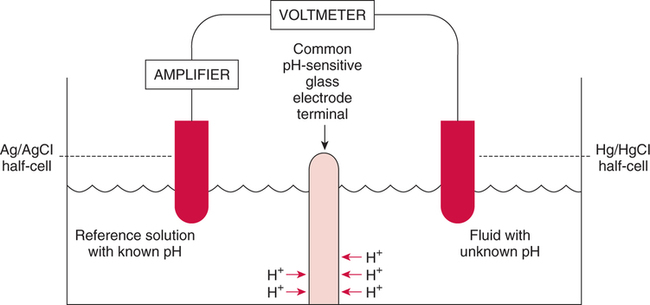
Physical Components
Sanz Electrode
PCO2 Electrode
Electrode Function
Accuracy of Electrodes
Parameters
Values
PO2
±3 mm Hg
PCO2
±1 mm Hg
pH
±0.01 units
TOTAL QUALITY MANAGEMENT
QUALITY ASSURANCE
Nonanalytical Error
Preanalytical Error
Postanalytical Error
Analytical Error
Calibration
QUALITY CONTROL
Statistics
Mean
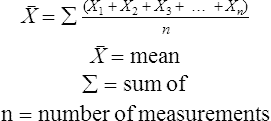 Equation 4-1
Equation 4-1
Standard Deviation
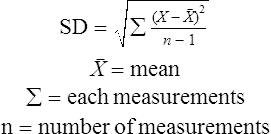 Equation 4-2
Equation 4-2![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Blood Gas Electrodes and Quality Assurance
Only gold members can continue reading. Log In or Register to continue

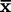 ), standard deviation (SD), and coefficient of variation (CV).
), standard deviation (SD), and coefficient of variation (CV).