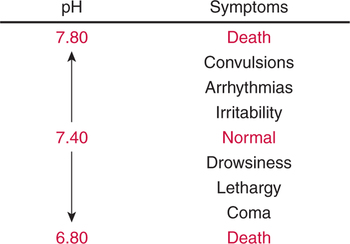
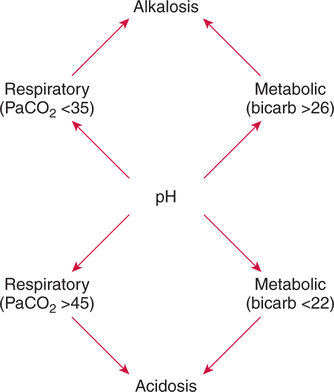
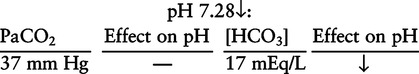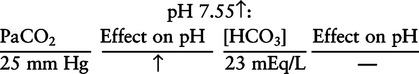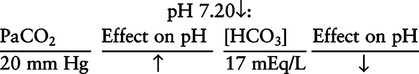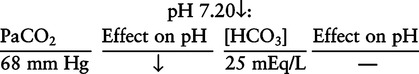There are three steps in this simple ABC approach to acid-base classification; these steps are shown in Box 2-1. First, Acid-base status (overall body conditions) is assessed by classifying the arterial pH. The arterial pH is the single best index of composite acid-base status in the body. Regarding the sequence of specific indices to be analyzed on a blood gas report, it is recommended that the pH be evaluated first as an index of overall Acid-base balance (Box 2-2). The PaCO2 (respiratory status) is evaluated next followed by [HCO3] (metabolic) evaluation. Assessment of both of these indices is necessary to complete both step 2 (Basic primary problem determination) and step 3 (Compensation assessment). As we will see later in this chapter, the clinician should then analyze the PaO2 (oxygenation classification) and consider it in respect to the FIO2 (i.e., the concentration of oxygen being inspired) and lung oxygen exchange efficiency. Again, Box 2-2 summarizes the sequence of indices to be evaluated. Low arterial pH has a generalized depressive effect on the human nervous system (Fig. 2-1).173 Symptoms may include drowsiness and lethargy. Regardless of the precipitating cause, a very low pH (i.e., pH <7.10) is often associated with coma. A pH of less than 6.80 for any extended period is generally considered to be incompatible with life. A condition that tends to cause acidemia is called an acidosis; therefore, all patients with acidemia must also have a primary acidosis. Similarly, a condition that tends to cause alkalemia is called an alkalosis; therefore, all patients with alkalemia must also have a primary alkalosis. For this reason, it is customary to simply call a low arterial blood pH acidosis and a high blood pH alkalosis (Table 2-1). A normal level of carbonic acid in the arterial blood corresponds to a PaCO2 level of 35 to 45 mm Hg, which is shown in Table 2-2. Indeed, PaCO2 can be used simply to evaluate and classify the respiratory acid-base status. Table 2-2 Classification of Respiratory Acid-Base Component In some cases, the adjective metabolic may actually be misleading because many non-respiratory acid-base disturbances (e.g., vomiting) do not involve changes in “metabolism.” In fact, some authors have suggested that the adjective metabolic should be replaced with the adjective non-respiratory.174 Nevertheless, the term metabolic is well ingrained in clinical medicine and is used throughout this text. Although various different metabolic indices have been advocated through the years, the plasma bicarbonate concentration [HCO3] (sometimes referred to as the actual bicarbonate) is probably the most widely used index and is seen on many clinical, professional, credentialling examinations. Initial blood gas classification examples in this text will include only the [HCO3] as a metabolic index to avoid confusion. Also, as mentioned previously, there are some uncommon situations when bicarbonate values may be misleading (discussed in Chapter 5). It is best for the novice to assume the bicarbonate is always a clear and concise indicator of metabolic status. Logically, one should first learn the general rules of classification and later address unusual exceptions. Bicarbonate represents the most important base in the blood plasma. The normal value for plasma bicarbonate in the arterial blood is 24 ± 2 mEq/L, which is shown in Table 2-3. Table 2-3 Classification of Metabolic Acid-Base Component The numeric value of bicarbonate decreases in response to either an accumulation of blood fixed acids or to a loss of blood base. This occurs due to blood acid-base buffering, which is discussed in a later chapter. Therefore, a decreased bicarbonate concentration indicates a non-respiratory condition that tends to cause acidemia (i.e., metabolic acidosis). Quantitatively, as shown in Table 2-3, a metabolic acidosis can be defined as a [HCO3] less than 22 mEq/L. Conversely, the numeric value of bicarbonate increases in response to increased blood base or to a fall in fixed acid levels. Thus, bicarbonate values higher than normal indicate metabolic alkalosis. Numerically, as shown in Table 2-3, metabolic alkalosis can be defined as a [HCO3] greater than 26 mEq/L. Classification of Primary Blood Gas Problems Table 2-4 highlights these key relationships, which must be memorized to identify the primary acid-base problem. Figure 2-2 illustrates the paths by which one can identify primary acid-base disturbances in patients with acidosis or alkalosis. In some less common cases, both respiratory and metabolic components may be pulling pH in the same direction. When there are two primary acid-base problems both pulling pH in the same direction, the problem is said to be “Mixed” or “Combined.” Table 2-4 Effect of PaCO2 and [HCO3] Change on pH Classification of Primary Blood Gas Problems Example 2-5 is one of the most common types of blood gases seen and also one of the most commonly misclassified, particularly by the novice. As previously stated, many inexperienced clinicians, after looking at pH and PaCO2, incorrectly classify this as simply respiratory alkalosis. The logic is that pH and PaCO2 are both abnormal; so the abnormal respiratory condition must be the cause of the overall pH acid-base disturbance. The error in this logic is failing to remember the relationship between PaCO2 and pH as shown in Table 2-4. Whenever the individual has a primary respiratory acid-base disturbance, the relationship between PaCO2 and pH must be inverse. This basic primary acid-base disturbance in this blood gas is a metabolic acidosis because a low [HCO3] tends to lower pH and indeed that explains the abnormally low pH. The low PaCO2 (respiratory alkalosis that tends to increase pH) is actually due to the normal compensatory response of the body, which is described later in this chapter. In summary, when determining primary problems, the key is to understand the relationships in Table 2-4. For the novice, it is recommended that, given a particular blood gas, only one of the metabolic indices should be classified to avoid confusion. Some unusual situations are discussed later in Chapter 5, when the two indices may not completely agree with each other. Obviously, if you are using [BE] as the metabolic index, the same rules apply. An elevated [BE] (e.g., +5 mEq/L) would increase pH and would be termed a metabolic alkalosis (see Table 2-3). A lower [BE] (e.g., -5 mEq/L) tends to lower pH and would be termed a metabolic acidosis.
Blood Gas Classification
ACID-BASE STATUS
pH Assessment
Clinical Significance
Clinical Manifestations of Abnormal pH
Classification of pH
Abnormal pH
BASIC (PRIMARY) ACID-BASE DISTURBANCE(S)
Respiratory Acid-Base Status
PaCO2 Classification
Classification
PaCO2 (mm Hg)
Normal respiratory component
35–45
Respiratory acidosis
>45
Respiratory alkalosis
<35
Metabolic Acid-Base Status
[HCO3] Classification
Normal Metabolic Status
Classification
[HCO3]*
[BE]*
Normal metabolic component
24 ± 2
0 ± 2
Metabolic acidosis
<22
< −2
Metabolic alkalosis
>26
> +2
Metabolic Acidosis
Metabolic Alkalosis
Identification of Primary Acid-Base Disturbances
Example 2-1
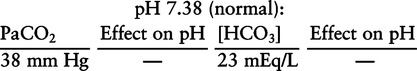
Index
Effect on pH
PaCO2 ↑
pH ↓
PaCO2 ↓
pH ↑
[HCO3] ↑
pH ↑
[HCO3] ↓
pH ↓
Example 2-4
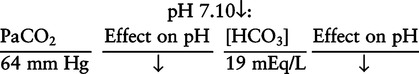
Base Excess [BE] Assessment
COMPENSATION ASSESSMENT
Blood Gas Classification