Benign Metastasizing Leiomyoma
Benign metastasizing leiomyoma (BML) is a rare disease of women, which is characterized by noninvasive, well-circumscribed tumors composed of differentiated smooth muscle cells, localized to sites other than the uterus.1–5 Lungs and lymph nodes6 are the most common sites involved, but BMLs have also been identified in the mediastinum,7,8 retroperitoneum,9 vascular channels,10 bone,11 heart,12 skeletal muscle,13 and soft tissues.14 Pulmonary BML has been associated primarily with uterine leiomyomas and has been mainly diagnosed in patients who have undergone uterine myomectomy or hysterectomy.1,4
The first report of BML, in 1939, described a 36-year-old woman who presented with dyspnea and wheezing. Chest X-rays showed multiple lung nodules. At autopsy, lymph nodes, uterus, and lungs showed nodules of smooth muscle cells with identical morphology15 and the term “metastasizing fibroleiomyoma of the uterus” was adopted. This term was later abandoned and the name BML was proposed because of the metastatic behavior of this disease.
Pathologically, BML lung nodules resemble hamartomas (the most common benign lung tumor), low-grade leiomyosarcomas, and nodules of proliferating smooth muscle cells.16 Pulmonary BML is usually asymptomatic and presents with either single or multiple lung nodules of varying sizes.17 The tumors do not appear to invade adjacent tissues.18 BML, however, represents a diagnostic and therapeutic challenge because of its pathogenesis and metastatic potential.
EPIDEMIOLOGY
The occurrence and prevalence of pulmonary BML is unknown. There are over 100 cases of BML reported in the literature but only a few studies reported more than one case. BML is a rare disease, found primarily in premenopausal women who have undergone surgical procedures for treatment of uterine leiomyomas.1,4,18 However, cases of lung BML have also been reported in women with no history of these surgical procedures.19 Leiomyomas, including BML of the lung, may be found in women and, to a lesser extent, in men and children.20 There is no ethnic or racial preference for BML and this differs from uterine leiomyoma, which is more frequent in African-American women.21 Cases of BML have been reported from countries all over the world4,22 including, but not limited to, the United States,19 Portugal,23 Brazil,24 China,25 India,14 South Korea,13 Japan,26 and Turkey.6
CLINICAL PRESENTATION
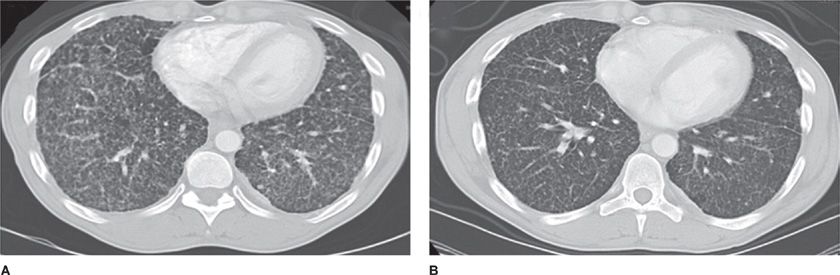
Most cases of lung BML have been identified as an incidental finding on imaging procedures performed for other purposes (Fig. 63-1).1 BML nodules may present in premenopausal women as unilateral or bilateral tumors with no specific lobar distribution.17,27 A number of different types of uterine tumors (e.g., leiomyoma, smooth muscle tumors of uncertain malignant potential, leiomyosarcomas, other smooth muscle tumors, endometrial stromal tumors) are associated with the diagnosis of BML. BML may present with respiratory symptoms including cough, wheezing, dyspnea, and chest pain.15,17,19,28,29 BML lung nodules have been detected in women in a few months to over 30 years after they have undergone uterine myomectomy or hysterectomy.30
Figure 63-1 Nodular structures in BML. High-resolution computed tomography (HRCT) shows multiple diffuse small bilateral nodules before (A) and after treatment (B). (Reproduced with permission from Taveira-DaSilva AM, Alford CE, Levens ED, Kotz HL, Moss J. Favorable response to antigonadal therapy for a benign metastasizing leiomyoma. Obstet Gynecol. 2012;119(2 Pt 2):438–442.)
PATHOLOGY
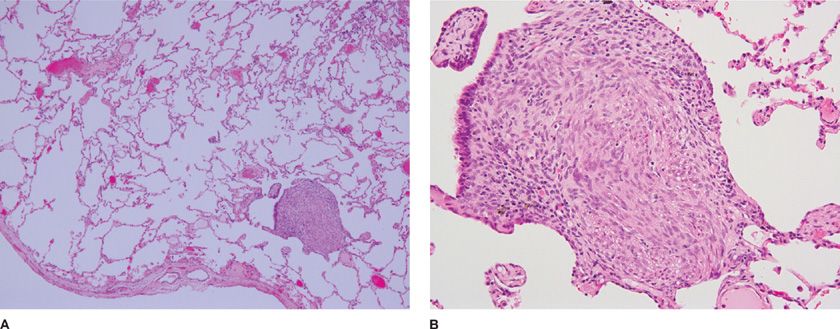
BML lung lesions consist of well-circumscribed nodules ranging in size from few millimeters to several centimeters in diameter.1,4 The lung tumors are composed of well-differentiated proliferative smooth muscle cells that form intersecting fascicles and show positive immunoreactivity toward actin, desmin, and caldesmon (Fig. 63-2).4,31 Most of these cells also react with antibodies against receptors for estrogen and progesterone.1,3,22
Figure 63-2 Histologic section of proliferative areas of lung BML. Tissue section shows proliferative smooth muscle cells forming fascicles characteristic of leiomyomas and BML. A, B. Show low- and high- resolution pictures of lung proliferative nodules. (Reproduced with permission from Taveira-DaSilva AM, Alford CE, Levens ED, Kotz HL, Moss J. Favorable response to antigonadal therapy for a benign metastasizing leiomyoma. Obstet Gynecol. 2012;119(2 Pt 2):438–442.)
Benign lung nodular or mass lesions may be either of epithelial or mesenchymal origin.32 BML lesions consist of well-differentiated smooth muscle cells, which form lung nodules with low cellularity, exhibiting low mitotic index and no nuclear atypism. The lung nodules do not invade the surrounding tissue and lack evidence of necrosis. Since most of the BML cases are discovered after hysterectomy or myomectomy, it would be important to identify the type of uterine cell causing the tumor. BML cells without a clearly distinct smooth muscle phenotype may exhibit unusual growth patterns.33 In general, BMLs have been classified as benign tumors of mesenchymal origin.
BML lesions contain vascular structures as determined by anti-CD34 antibody reactivity within nodules adjacent to vessels. BML lesions express variable levels of p53, but the role of this tumor suppressor in BML cells has not been reported.31 BML lung lesions also show reactivity with antibodies against proliferating cell nuclear antigen (PCNA). In contrast to what is seen in leiomyomatous hamartoma, most of the histopathologic sections of lung BML are not reactive with the monoclonal antibody Human Melanoma Black-45 (HMB-45), which recognizes Pmel17,31 a melanosomal protein expressed in cells from lung hamartomas, PEComas (perivascular epithelioid cells), and lymphangioleiomyomatosis (LAM).34 BML nodules are in most of the cases not reactive to antibodies against EMA, CD10, CD117, TTF-1, BCL-2 GPAP, calretinin, and cytokeratin chromogranin. S-100 is expressed at very low levels in BMLs. PEComas of the uterus with pulmonary metastases have a similar presentation to BML but the proliferating smooth muscle cells are mostly nonreactive to HMB-45.19,35,36 Thus, BML lung nodules appear to have distinct pathologic characteristics.
It has been proposed that leiomyomas should be classified as benign or malignant based on the number of mitotic figures. If 10 mitotic figures per 10 high power field (HPF) are present, the tumor is classified as a neoplasm and if there are more than 5 mitotic figures per 10 HPF it should be considered a leiomyosarcoma. Benign lesions should have less than 5 mitotic figures per HPF.37 The fact that BML lesions contain less than 5 mitotic figures per HPF, identifies them as a benign tumor.
It is possible to identify cancer cells based on their molecular phenotype,38,39 however, the molecular characteristics of BML cells remain unknown. Expression of the micro-RNA 221 (miR-221), which has been correlated with different malignancies,40 appears to differentiate leiomyosarcoma from BML.41 Molecular assays of X-chromosome inactivation have shown that it is very likely that the lung and uterine cells have a similar origin.42 BMLs proliferate without telomeric changes, suggesting that their proliferative behavior is independent of telomeric attrition as is the case in other malignant diseases.43,44 Cytogenetic studies of BML lung tumors have shown that the cells have abnormalities in several chromosomes. BML tumors from five cases showed 19q and 22q terminal deletions.45 Interestingly, a single case showed multiple chromosomal deletions from cells isolated from different metastatic sites.46 Rearrangement of the 6p21 region was correlated with changes in the high mobility group A1 gene (HMGA1). Chromosomal translocation and mutations have been associated with HMGIC in leiomyomas but they have not been identified in the leiomyomas present in BML patients. It has been possible to correlate the proliferative behavior of BML cells with chromosomal translocations present in other noninvasive tumors.45–47
Some of the factors potentially involved in the development of uterine leiomyoma are basic fibroblast growth factor (bFGF), transforming growth factor-beta (TGF-β) and granulocyte macrophage colony–stimulating factor (GM-CSF),48 although a role for these factors in the pathogenesis of BMLs has not been defined. Analysis of BML tumor cells from different sites suggests a clonal origin.42 The balanced karyotype of BMLs is consistent with the findings seen with leiomyomas.49 The clonality of these tumors should be interpreted with caution due to the fact that the founding mutation has not been identified in any case.
PATHOGENESIS
BMLs are considered mesenchymal tumors not mixed tumors of the uterus. Although there are different types of leiomyomas (e.g., mitotically active, cellular, hemorrhagic cellular, atypical, epitheliod, myxoid, vascular, lipoleiomyomas), the type of leiomyoma associated with BML has not been determined. Hamartomas, the most common tumor of the lung, are seen in both genders. PEComas of the uterus with pulmonary metastases may have a similar presentation to BML but these tumors express Pmel7, and are recognized by the monoclonal antibody HMB-45.
The source of the cells that form the lung nodules remains unknown, but due to the strong association with uterine leiomyomas, it is believed that the main source of the cells is the uterus. It has been proposed that those cells responsible for the formation of pulmonary BML are derived from (a) low-grade tumors; (b) cells dislodged from uterus at the time of myomectomy and hysterectomy; (c) metastasis from uterus or an unknown site; (d) proliferation of lung smooth muscle cells; and/or (e) simultaneous-independent development of multiple leiomyomas.
 LOW-GRADE TUMORS
LOW-GRADE TUMORS
Although other tumors may occur in patients with BML including leiomyosarcoma, adenocarcinoma,50 breast carcinoma, and skeletal muscle tumors, their association with BML is not clear. Leiomyomas of the esophagus, lung, and uterus are found in association with multiple endocrine neoplasia type I (MEN1) but loss of heterozygosity for MEN1 has not been investigated in BML, as is the case in many MEN cases.51 Tumors characterized as low-grade leiomyosarcomas and high-grade leiomyosarcomas do not appear to metastasize to the lung; these tumors occur mainly in the abdominal cavity.52 It has also been proposed that BML could be an intermediate tumor stage leading to a malignant leiomyosarcoma7 but high-grade leiomyosarcomas involving the lung reappear in a short period of time following surgery.52 Low-grade leiomyosarcomas do not express progesterone and estrogen receptors as is the case in BML. Thus, it is very unlikely that BMLs arise from low-grade malignancies.
 CELLS DISLODGED FROM UTERUS AT THE TIME OF MYOMECTOMY AND HYSTERECTOMY
CELLS DISLODGED FROM UTERUS AT THE TIME OF MYOMECTOMY AND HYSTERECTOMY
Since pulmonary BML has been associated with hysterectomy and myomectomies, it has been postulated that some of the uterine cells move into the blood circulation at the time of surgery and migrate to the lung where they remain and grow at a slow rate.18 Lung BML has been associated with tumors in the retroperitoneal cavity,9 pelvis, and the para-aortic lymph nodes arguing against the passive passage of cells from uterus to lung.18 The presence of BML tumors in the pelvic region is not in agreement with the concept that uterine cells became dislodged and seeded the lung. Although most cases of BML report multiple pulmonary tumors after hysterectomy, few cases describe the occurrence of nodules before hysterectomy.53 BML has been correlated with endometriosis as BMLs and endometriosis have a similar course and may develop after hysterectomy or myomectomy.3,4 Estrogen and progesterone receptors are found in lung BML cells and uterine leiomyomas, which are the most likely source of the cells. However, it is not clear if lymphatics or blood vessels are involved in the metastatic process leading to pulmonary BML.
 METASTASIS FROM UTERUS OR UNKNOWN SITE
METASTASIS FROM UTERUS OR UNKNOWN SITE
Due to the fact that BMLs are present in multiple sites, it is possible that either uterine smooth muscle cells or cells from other nonuterine sites metastasize to the lung. Forty percent of leiomyomas or myomas possess specific chromosomal abnormalities.49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree