Introduction and historical perspective
Percutaneous balloon mitral valvuloplasty (PBMV) represents part of an evolution from early, primitive cardiac surgical techniques to the modern era of percutaneous valve interventions. This evolution began with Elliot Cutler advancing a knife retrograde through the apex of the left ventricle of a beating heart in 1923.1 Neither he nor Henry Suttar, who performed what today would be considered an antegrade fingertip commissurotomy in England 2 years later,2 received the expected accolades. In part this reflected disagreement over the role of mitral valve obstruction in defining the spectrum of mitral stenosis (MS). Sir Thomas Lewis’ statement that valvotomy was based on an “erroneous idea, namely that the valve is the chief source of the trouble”3 has few proponents in the modern era. Nevertheless, because of the broad spectrum of cardiac and non-mitral valvular involvement resulting from rheumatic carditis and valvulitis,4 relieving mitral valve obstruction largely palliates rather than cures patients with MS.
Some 20 years after the initial attempts to relieve mitral obstruction, the surgical experience in World War II battle-field hospitals with closed heart procedures led to the application of newly learned techniques to civilian use. Although early results were confounded by significant morbidity and mortality, closed mitral valvotomy became a routine procedure for severe MS by the 1950s and 1960s, and is still performed in many parts of the world where the disease is endemic and medical facilities are limited. Large series5,6 have claimed good long-term results, but lack of systematic follow-up or comprehensive objective data obscures the actual restenosis rate and survival. In a Mayo Clinic retrospective analysis7 there was a 79% 10-year and a 55% 20-year survival rate with reoperation in 34% by 10 years; however, nearly a quarter of patients were lost to follow-up and severity of disease at baseline could only be estimated. A more recent evaluation of a 36-year experience included a 44% rate of repeat surgery by 12 years.8 Open commissurotomy, with the potential advantages of direct vision, has supplanted closed procedures in industrial nations.9 Controversy remains as to its superiority,10–12 with the advantages of direct vision favoring cases where thrombus is present. The relative role of open commissurotomy versus mitral valve replacement with or without chordal preservation has been explored with somewhat equivocal results.13 Mitral valve replacement has higher periprocedural and postoperative event rates including mortality, but superior freedom from repeat mitral valve intervention; when the chordae to at least one leaflet are preserved, the outcomes of mitral valve replacement are superior (Level C).
Following the initial percutaneous structural heart disease interventions of Rashkind with balloon atrial septostomy,14 a device designed by Inoue to inflate sequentially across the septum (Fig. 57.1) was instead adapted for PBMV.15 After initially demonstrating its ability to split fused commissures in the operating room,16 the first percutaneous PBMV was performed in 1982.17 After several small series demonstrated feasibility,18,19 a number of alternative techniques were developed, including large single balloons,18 two cylindrical balloons used in parallel over two guide-wires20 through a single or two transseptal punctures,21 a monorail technique for using two balloons,22 a retrograde technique that avoided the transseptal approach23 and a percutaneous metal commissurotome.24 The initial technique and device developed by Inoue, with scant modification, remains the predominant (and only US FDA approved) approach.
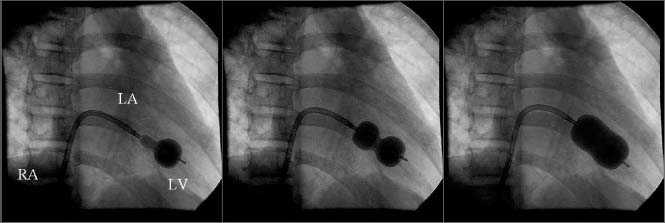
Figure 57.1 Deployment of the Inoue balloon via the transseptal approach. The catheter has been advanced across the interatrial septum from the right atrium (RA) into the left atrium (LA) and then into the left ventricle (LV) across the stenotic mitral valve. The balloon is designed to inflate distally first (left frame), then to be pulled back against the mitral valve after which the proximal portion inflates (center frame), centering the balloon across the valve. As additional volume is injected into the balloon, full inflation occurs, ideally causing splitting of the commissures (right frame).
PBMV typically results in a doubling of the mitral valve area (on average from 1.0 cm2 to 2.0cm2) with a 50% reduction in gradient and an 80–95% success rate, the latter defined as the mitral valve area increasing to >1.5cm2 and left atrial pressure decreasing to <18mmHg without major complications.25 Overall, the results are highly operator dependent with a steep learning curve.26,27 As a result, the ACC/AHA guidelines25 emphasize that the decision to proceed to PBMV must take into consideration the experience of the operators involved (Class I, Level B).
Mechanism of balloon commissurotomy and Pathophysiology
In general, balloon valvuloplasty, regardless of the valve being dilated, has three possible mechanisms for relief of obstruction: stretching of the valve orifice, splitting of fused commissures, and cracking of valvular calcifications. In the case of PBMV, the mechanisms28 responsible for the benefits seen arise from the substantial radial force29 exerted by the enlarging balloon. This stretches the mitral annulus, has the capacity to split fused commissures, and occasionally results in cracking of calcifications. The stretching mechanism has been described in the intraoperative setting.30 The splitting of commissures31 and cracking of calcification32 have been demonstrated by direct observation in excised valves. The largely successful nature of PBMV is derived virtually exclusively from commissural splitting of rheumatically fused leaflets.33 Balloon dilation procedures dependent on the other two mechanisms, such as balloon valvuloplasty for calcific aortic stenosis34 or bioprosthetic valves,35 have poor short-and long-term results. Thus non-rheumatic MS secondary to calcification of the mitral annulus, leaflets or subvalvular apparatus, such as seen with elevated hyperparathyroid levels (e.g. patients on dialysis), lends itself poorly to balloon dilation (Level C).
Successful splitting of the commissures results in decreased gradient and improved mitral valve area, and typically leads to significant clinical and hemodynamic improvement. This occurs progressively, with reduction in gradient, left atrial pressure and pulmonary artery pressures continuing over a 3-year period.36 It is important to consider that relief of mitral valve obstruction will typically increase left ventricular volume loading secondary to facilitated left atrial emptying. In the relatively underfilled ventricle seen with “pure” MS this is typically well tolerated, although some degree of left ventricular dysfunction in patients with rheumatic heart disease may be present even without concomitant valvular regurgitation. In patients with concomitant mitral or aortic insufficiency, the left ventricle may become volume loaded after PBMV. Thus, although the gradient may be substantially reduced, left heart filling pressures may rise and be accompanied by failure of clinical improvement or actual deterioration.
Indications
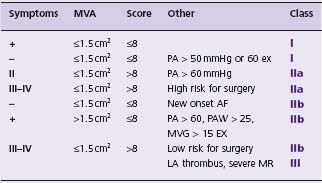
Balloon valvuloplasty is primarily indicated for moderate to severe MS (Table 57.1) and is the procedure of choice for symptomatic patients if the valve anatomy is suitable and there is neither left atrial thrombus nor greater than mild mitral insufficiency (Class I, Level A). The guidelines incorporate evidence that PBMV is beneficial for patients with MS and pulmonary hypertension (Class I) or atrial fibrillation (AF) (Class II, Level B). The guidelines have been expanded to include mild MS under limited circumstances (Class II, Level C).
Table 57.1 Indications for percutaneous balloon mitral valvuloplasty, incorporating modifications from the 2006 ACC/AHA guidelines.25 Note that the presence of pulmonary hypertension in asymptomatic patients is now a Class I indication. The effect of exercise has been incorporated. Pulmonary hypertension is assumed to be secondary to mitral stenosis. Where the guidelines specify valve morphology favorable for PBMV, the table lists an echo score ≤ 8 using the MGH scoring system. It should be noted that a boundary effect likely exists, and the scoring system has significant limitations so the guidelines do not specify either a specific scoring system or an absolute threshold. AF, atrial fibrillation: LA, left atrium; MR, mitral insufficiency; MVA, mitral valve area; PA, pulmonary artery systolic pressure.
Preprocedure evaluation and prediction of outcomes
A variety of anatomic and physiologic factors determine suitability for PBMV. Anatomic features that correlate with procedural success as well as long-term outcomes have been described since the early development of PBMV.37 These include characteristics of the valve leaflets, annulus and subvalvular apparatus. Although there are major limitations, the most commonly used has been a scoring system described by Wilkins and colleagues38 that incorporates thickening, mobility and calcification of the valve leaflets as well as disease of the subvalvular apparatus (Table 57.2). This, the so-called MGH (Massachusetts General Hospital) scoring system, assigns up to four points for the most severe disease for each characteristic, with a maximum of 16 points. Ideal valves have a score of eight or less, and echo scores >12 have been predictive of poor short- and long-term outcomes39 (Fig. 57.2) (Level B).
Table 57.2 The Wilkins–Weyman echocardiographic scoring system of mitral valve characteristics
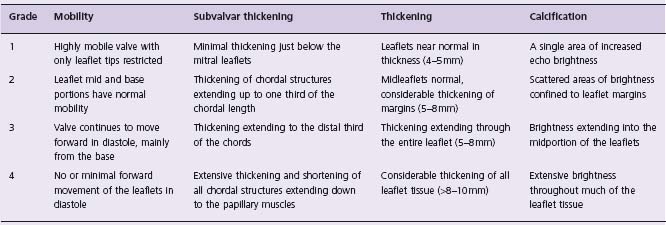
The total echocardiographic score was derived from an analysis of mitral leaflet mobility, valar and subvalvar thickening, and calcification which were graded form 0 to 4 according to the above criteria. The total possible score ranges from 0 to 16.
(Reprinted by kind permission of the BMJ Publishing Group from reference.)38
Figure 57.2 Survival free of repeat balloon dilation or mitral valve replacement in 879 patients undergoing percutaneous balloon mitral valvuloplasty with echo scores of ≤8, 9–11, and ≥12. (Reproduced from Palacios39 with permission of the American Heart Association, Inc.)
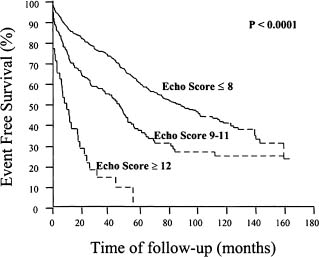
Intermediate and long-term event-free survival has correlated well with the echo score, as well as the immediate postprocedure hemodynamics39–42 (see Fig. 57.2). Five-year event-free survival is negligible in patients with echo scores of 12 or greater, but approximately two-thirds in those with favorable valve morphology (Level B).
While the correlation between the echo score and initial as well as long-term outcomes has been good, the MGH scoring system has been criticized because it is semiquan-titative, does not include important anatomic elements such as the nature of commissural fusion, and because one element of the score, leaflet mobility, correlates more strongly with outcome (r value = 0.67) than the complete score. Valve calcification alone predicts a fourfold increase in cardiac complications and a 26% increase in 6-year mortality.43 Anatomic features such as a funnel-shaped subval-vular apparatus and eccentric commissural fusion44 are not included in the score, nor is severe fusion and calcification of the commissures45; all are strong negative predictors of outcome.46 In the case of calcification, valve area varied inversely in 223 patients: 0.9 cm2 in those with no calcification versus 0.8, 0.7 and 0.6 cm2 respectively in those with grade 1, 2 and 3 calcium (P < 0.05).47 A number of other scoring systems have been proposed, some of which appear to have a strong correlation with outcomes,48 including an echocardiographic system assessing characteristics of leaflets and the subvalvular apparatus that correlates with PBMV-induced mitral regurgitation.49 Perhaps the most compelling reason for routinely deriving the MGH echo score is to allow for comparison with known data: most PBMV trials have incorporated this system to define baseline severity of disease. As such, an echo score ≤ 8 is commonly interpreted as representing the ideal valve anatomy for PBMV described in the ACC/AHA guidelines25 (see Table 57.1).
Besides anatomic features, a number of other factors appear to predict acute and longer term results. The presence of AF is an independent risk factor for adverse outcomes in several datasets.50 A recent analysis of a 531-patient cohort with long-term follow-up demonstrated inferior acute results (1.87 v 2.0 cm2 mitral valve area (MVA) and higher restenosis rates (33% v 23% at 10 years, 66% v 54% at 15 years)51 in patients with AF. Age39,52 and severity of stenosis as defined by hemodynamics and functional class53 correlate with outcomes based on multivariate analyses (Level C).
Physiologic determinants of outcome may include the presence of left ventricular volume loading or dysfunction with or without concomitant valvular or myocardial disease. PBMV in the setting of more than mild mitral insufficiency is an independent predictor of adverse events at long-term follow-up.39 Increase of mitral regurgitation by one grade is common after PBMV, occurring in some 33% of patients; an additional 13% increased by two grades or more.54 Superimposed on pre-existing mitral insufficiency, developing post-procedure mitral regurgitation of 3+ or greater is therefore a significant risk, the latter being an independent predictor of adverse outcomes as well.39 In contrast, PBMV in the setting of mild to moderate aortic insufficiency (AI) is usually well tolerated. Comparing outcomes in 315 patients with mild or moderate AI versus 361 patients without AI, the only significant difference in outcomes was a higher rate of aortic valve replacement in patients with moderate AI, albeit at a mean of 4.1 years after PBMV55 (Level C).
Transesophageal echocardiography
Routine preprocedure transesophageal echocardiography (TEE) has become the standard of care and has been raised to a Class I, Level C indication. Essential is the increased sensitivity for detection of left atrial thrombus,56 as well as aspects of mitral valve morphology and regurgitation; however, the subvalvular apparatus is better interrogated with transthoracic echo. It should be noted that left atrial thrombus occurs outside the appendage in 43% of patients with rheumatic heart disease.57 Although left atrial thrombus is primarily seen in patients in AF, it has been reported in approximately 2% of severe MS patients in sinus rhythm.58 Based on a study of 100 patients referred for PBMV, there is a significant increased risk of left atrial thrombus in the presence of spontaneous echo contrast.59 However, with successful resolution of the gradient, and perhaps with the detergent effect of any periprocedural mitral insufficiency, resolution of spontaneous echo contrast corresponds with relief of mitral obstruction.60 An increasing body of evidence has also demonstrated increased coagulability in patients with mitral valve stenosis.61 Finally, other structural abnormalities such as the presence of vegetations or ruptured chordae can be detected by TEE with much higher sensitivity than surface echo.
Cardiac catheterization to assess hemodynamics is indicated only if the non-invasive evaluation is equivocal in assessing valve anatomy and physiology, ventricular function and pulmonary hypertension (Level C). Patients with angina, objective evidence of ischemia or known coronary artery disease should have coronary angiography before PBMV; coronary angiography for risk factors alone is not required (Level C). A new element in the guidelines is a Class IIb indication for so-called “drive-by” coronary angiography regardless of whether patients meet these criteria if they do require a hemodynamic study because of equivocal non-invasive data; there is no evidence base for this recommendation, however (Level C).
The severity of MS can be determined by multiple methods including gradient, valve area as derived from multiple techniques, and finally the pulmonary artery pressure. It is important to consider that each technique is fraught with significant potential for inaccuracy. Importantly, the gradient is highly flow dependent, and is thus a poor proxy for severity of disease. It is particularly likely to lead to overestimation of severity of MS in the setting of poor heart rate control. Patients with new onset of AF are highly prone to having the extent of MS judged severe when it is mild or moderate, since these patients usually have poor initial rate control combined with loss of the contribution of atrial contraction to atrial emptying. Because tachycardia disproportionately shortens the dia-stolic filling period, they also have disproportionately high gradients and far worse symptoms than their MS would otherwise warrant. Inappropriate intervention in these patients, when heart rate control or cardioversion would suffice, is a classic error in management of MS. In contrast, excessive bradycardia or dehydration (such as occurs when a patient is maintained without fluids pending catheterization for an extensive period of time) can lead to gross underestimation of severity of MS. The use of pulmonary artery wedge pressures as proxy for left atrial pressure leads to overestimation of the gradient and underestimation of the mitral valve area because of the delayed decompression of wedge pressure across the high resistance of the pulmonary vascular bed.62
The ACC/AHA guidelines specifically cite left atrial thrombus and greater than mild mitral regurgitation as absolute contraindications and suggest that severe calcification and severe subvalvular disease are relative contraindications.
Left atrial thrombus
Several studies have explored chronic oral anticoagulation followed by repeat TEE to assess for thrombus resolution prior to PBMV. The rate of resorption is highly variable; the ACC/AHA guidelines recommend anticoagulation for 3 months. However, a prospective survey found resorption at 6 months in only one-quarter of 219 candidates for PBMV63; resorption was predicted by absence of spontaneous echo contrast, small thrombus size, and anticoagulation to an INR of 2.5 or greater. If clot remains present, we generally would avoid PBMV in favor of surgery since avoiding the left atrial appendage with guidewires and balloons, while usually feasible, takes significant additional skill, and even in the best of hands cannot be guaranteed. Nevertheless, Chen and colleagues64 have described a large series of patients with apparent organized left atrial appendage clot who underwent uncomplicated PBMV despite documented left atrial appendage thrombus. We believe that clot elsewhere in the left atrium remains an absolute contraindication, although novel algorithms such as placing distal protection devices in both internal carotid arteries have been described65 (Level C).
Mitral regurgitation
A comparison of 25 patients with moderate mitral regurgitation and 25 age-and gender-matched patients with mild or no regurgitation demonstrated an increased incidence of severe insufficiency post procedure in the former.66 However, these patients had much higher echo scores and twice as frequently had severe calcification. While 20% of those with initially moderate mitral regurgitation developed severe regurgitation, hemodynamic improvement overall was similar, as was the incidence of postprocedure mitral valve replacement. Even patients with mild mitral regurgitation tend to have less favorable anatomy at baseline and lower event-free survival but a similar success rate67 (Level C).
Severe calcification
Balloon dilation of a symmetric fused and severely calcified and essentially immobile mitral apparatus as well as attempted dilation of a severely calcified mitral annulus are largely futile. Thus patients with symmetric severely fused commissures may not respond at all.45,68 In contrast, those with asymmetric calcification are prone to leaflet tearing or rupture due to asymmetric application of force to the less diseased commissure or leaflet.69 While high echo score alone does not predict the occurrence of severe post-PBMV mitral regurgitation,70 one component, severe calcification, does.71 In general, greater degree of calcification predicts less successful improvement in valve area, higher peripro-cedural complications, and lower event-free survival.43 Nevertheless, when the risk of surgery is prohibitive, growing experience with predominantly elderly patients with high echo scores and poor overall morphology has shown moderate improvement in hemodynamics and palliation of symptoms at the cost of high morbidity and mortality72 (Level C).
Except for a small cohort of patients, virtually all PBMV has been performed via the antegrade transseptal route. The retrograde approach avoids the need for transseptal puncture, an advantage that is offset by the need to introduce relatively large hardware into the arterial circulation and to advance a balloon retrograde across the submitral apparatus over a guidewire, potentially causing entrapment in the subvalvular apparatus. Nevertheless, the success rates and intermediate term follow-up of retrograde PBMV have been comparable to the antegrade approach (88% success in a 441-patient cohort) with the usual finding of outcomes correlating with the MGH echo score.73 A small, 72-patient, single-site non-randomized comparison found no significant difference in outcomes between the antegrade Inoue technique and retrograde approaches except for increase in mitral insufficiency with the latter, but the study was underpowered to assess trends in postprocedure adverse events.74
The Inoue technique is virtually unchanged since the mid-1980s. In a review of 19 series reporting results in a total of 7091 patients, the overall success rate was 93%.75 Success was variably defined, and in some studies the definition included patients with severe iatrogenic mitral insufficiency, atrial septal defect or embolic events, but typically included a doubling of valve area. The principal features of the Inoue device are a balloon with a stretchable distal shaft to facilitate transseptal passage, a nylon mesh over a latex balloon that allows for variable inflation of the distal and proximal portions to promote straddling of the mitral valve (see Fig. 57.1), and a compliance curve that allows the balloon to dilate predictably over at least a 4 mm range of sizes. The device lends itself to a stepwise dilation technique, with repetitive inflations at progressively increasing balloon sizes.76 After each inflation, residual gradient, hemodynamics including blood pressure and left atrial pressure, and echocardiographic data are assessed; if residual MS is still substantial and mitral insufficiency has not increased, repeat inflation with the balloon inflated to a larger size is performed. No formal evidence base exists comparing this stepwise technique to a single inflation at the calculated optimal balloon size based on patient height and body surface area. The compliance curve of the Inoue balloon results in an exponential rise in radial force within a mm of its maximal size and is a risk factor for post-PBMV mitral insufficiency77 (Level C).
PBMV is typically performed with continuous TEE or, more recently, intracardiac echocardiographic guidance.78
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


