INTRODUCTION AND SURGICAL HISTORY
Atrioventricular septal defects (AVSDs) comprise a spectrum of lesions characterized by deficient atrioventricular (AV) septation and a variety of AV valve anomalies. AVSDs occur due to failure of fusion of the embryonic endocardial cushions. AVSDs represent 4% to 5% of congenital heart defects. Other terms have been used to describe this group of defects, including “AV canal defect,” “persistent common AV canal,” “atrioventricular defect,” and “endocardial cushion defect.” For the purposes of this chapter, the term “AVSD” will be used. The severity of AVSD is determined by many factors, including the size of atrial and ventricular level shunts, the extent of AV valve abnormalities, the presence of associated cardiac anomalies, and discrepancy in ventricular sizes.
The first surgery for AVSD was performed by Clarence Dennis in 1951 at the University of Minnesota and was the world’s first use of a cardiopulmonary bypass machine. The case involved a 6-year-old girl who was intraoperatively discovered to have an AVSD. She unfortunately died; her defect was deemed “unrepairable.” At that time, the lack of understanding of surgical anatomy made repair extremely challenging. Gian Carlo Rastelli, an Italian born research surgical associate at Mayo Clinic, recognized the important need for better understanding of surgical anatomy. In 1966, Rastelli and John Kirklin at Mayo Clinic published a detailed anatomic review of AVSD resulting in the “Rastelli” Classification for AVSD. In 1968, Rastelli reported the surgical outcomes of AVSD and described novel reparative techniques. This study revealed a 40% reduction in hospital mortality. His novel surgical techniques revolutionized operative management of AVSD. Rastelli later went on to describe the “Rastelli procedure” for relief of pulmonary outlet obstruction in patients with transposition, VSD, and pulmonary stenosis. Despite his enormous contributions to the field, he was diagnosed with Hodgkin lymphoma at age 31 years and eventually died at age 36. Strikingly, Rastelli kept the severity of his illness secret from his peers and it was during this time when he produced these immense contributions to the understanding of AVSD. Thanks in large part to the foundation of knowledge that Rastelli established, long-term survival after surgical repair for AVSD has improved and 20-year survival of 95% is now reported. However, reoperation awaits 15% to 25% of patients because of progressive left AV valve regurgitation or development of left ventricular outflow tract (LVOT) obstruction. Therefore, long-term postoperative echocardiographic surveillance is required in all patients with AVSD.
TERMINOLOGY AND CLASSIFICATION
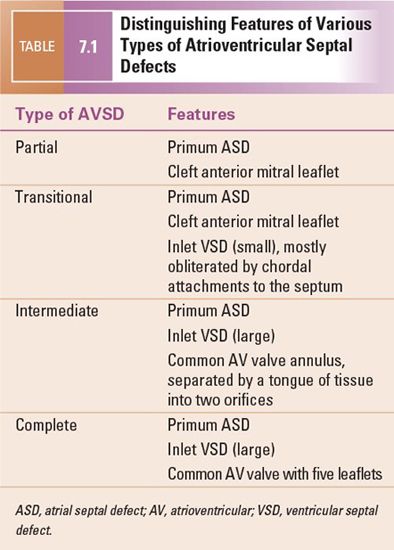
Many classification schemes have been used to describe AVSDs, resulting in confused terminology (Figs. 7.1 and 7.2). There is general agreement that AVSDs can be subdivided into two forms: complete and partial. Complete AVSD is characterized by a primum atrial septal defect (ASD) that is contiguous with an inlet ventricular septal defect (VSD), and the presence of a common AV valve. The common AV valve is composed of five leaflets: anterior and posterior bridging leaflets, an anterior leaflet (present on the right side), and right and left lateral leaflets.
The typical partial AVSD is distinguished from complete forms of the malformation by the absence of an inlet VSD. The anatomic hallmarks of partial AVSD are the primum ASD and a cleft in the anterior mitral valve leaflet. A valvular “cleft” differs from a commissure in that a cleft has no cords arising from the edges and is without subjacent papillary muscle tissue. This type of AVSD has distinct mitral and tricuspid valves, each with a complete and separate annulus.
Two other subtypes, the intermediate and transitional forms, have been described. Intermediate AVSD is considered a variant of complete AVSD and is characterized by a single AV valve annulus that is divided by a tongue of tissue into right and left orifices. Because of the single annulus, it is preferred to use the terms “right and left components of the common AV valve” in complete and intermediate AVSDs rather than “tricuspid” and “mitral” valves.
A transitional AVSD has two separate AV valve annuli and is considered a variant of partial AVSD. In addition to a primum ASD and a cleft mitral valve, there is a small inlet VSD that is often restricted or obliterated by dense chordal attachments of the AV valves to the crest of the ventricular septum. Complete and intermediate AVSDs have the physiology and clinical features of an ASD and a VSD. In contrast, partial and transitional AVSDs have the clinical picture of a large ASD (Table 7.1).
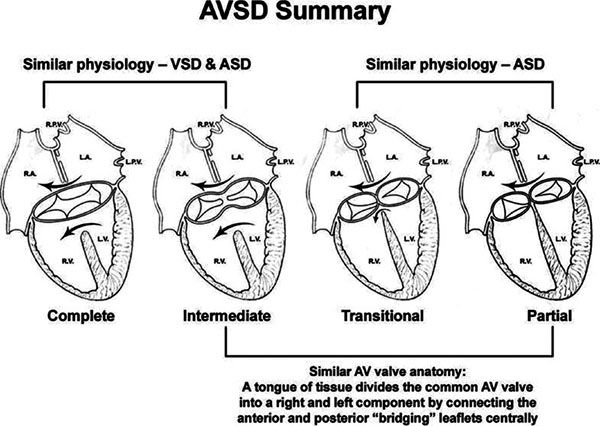
Figure 7.1. Summary of atrioventricular septal defects (AVSDs). Anatomic and physiologic similarities between the different forms of AVSD are illustrated. Complete AVSD has one annulus with a large atrial septal defect (ASD) and a large ventricular septal defect (VSD). Intermediate defects (one annulus, two orifices) are a subtype of complete AVSD. Complete AVSD has physiology of a VSD and an ASD. Partial AVSD has physiology of an ASD. Transitional defects are a subtype of partial AVSD in which a small inlet VSD is also present. Partial defects and the intermediate form of complete AVSD share a similar anatomic feature: a tongue of tissue divides the common AV valve into distinct right and left orifices. AV, atrioventricular; LA, left atrium; LPV, left pulmonary vein; LV, left ventricle; RA, right atrium; RPV, right pulmonary vein; RV, right ventricle.
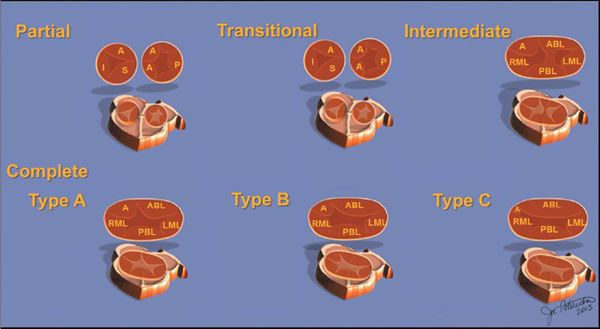
Figure 7.2. Spectrum of atrioventricular septal defects (AVSD). The diagrams on the upper row illustrate the spectrum of AVSDs. Partial (upper left), transitional (upper middle), and intermediate (upper right) forms of AVSD. The spectrum of complete AVSD can be subclassified into Rastelli types A, B, and C, illustrated in the lower row of diagrams. A, anterior leaflet; AB, anterior bridging leaflet; I, inferior leaflet; LML, left mural leaflet; P, posterior leaflet; PBL, posterior bridging leaflet; RML, right mural leaflet; S, septal leaflet (with permission of Mayo Foundation).
CHARACTERISTIC ANATOMIC FEATURES OF AVSDS
A number of characteristics are shared by all forms of AVSDs. These features aid in making the echocardiographic diagnosis of AVSD and have important clinical implications (Table 7.2).
Level of Atrioventricular Valve Insertion
In normal hearts, the tricuspid valve inserts onto the ventricular septum more apically than the mitral valve. With this “offset” in the level of insertion, a portion of septum, called the AV septum (Fig. 7.3A), separates the right atrium (RA) and the left ventricle. In AVSDs, both right and left AV valve components insert at the same level, and this is best appreciated from an apical four-chamber view. Apical four-chamber imaging demonstrates the crux of the heart, which has been referred to as the most reliable and consistent intracardiac landmark. A unifying feature of all forms of AVSDs is the complete absence of the AV septum (Fig.7.3B).
Unwedging of the Aortic Valve
In normal hearts, the aortic valve is “wedged” between the mitral and tricuspid valves (Fig. 7.4). In AVSDs, the aortic valve is “sprung” and displaced anteriorly. This contributes to elongation of the LVOT. This is best appreciated from the parasternal long-axis and subcostal outflow views.
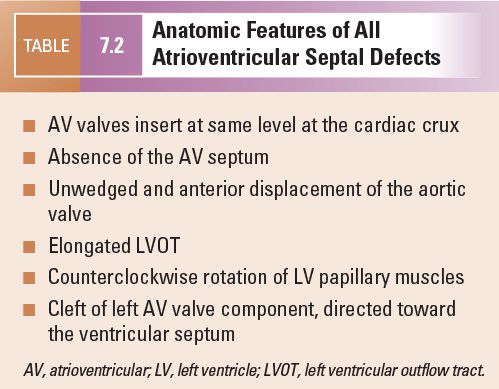
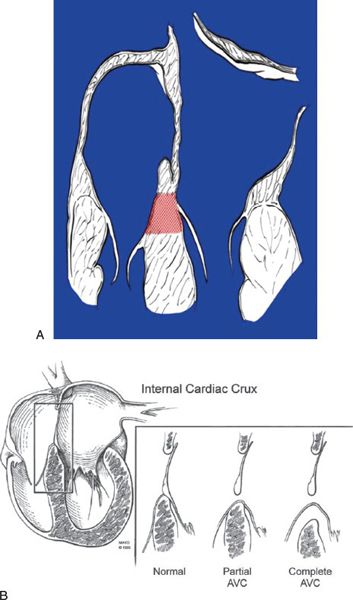
Figure 7.3. Atrioventricular septum (AVS). A: Diagram of the AVS (shaded area) in the normal heart (four-chamber view). The AVS lies between the right atrium and the left ventricle. The interatrial septum is above and the interventricular septum is below the AVS. The septal tricuspid leaflet normally inserts at a lower (more apical) level than the anterior mitral leaflet. B: Line drawing demonstrating the deficiency of the AVS in all forms of AVSD (with permission of Mayo Foundation).
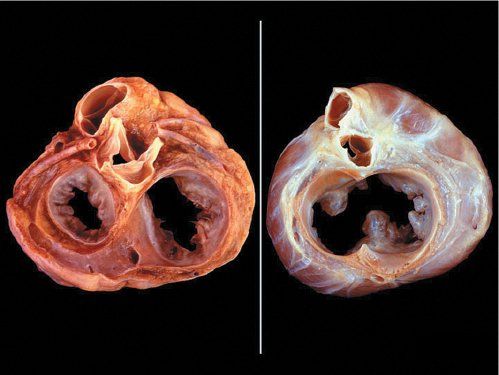
Figure 7.4. Unwedged aortic valve. Left: Normal pathologic specimen cut in short-axis at the base demonstrating where the atrioventricular junction has a “figure eight” configuration. Right: Similar projection in a heart with an atrioventricular septal defect (AVSD) where the atrioventricular junction is “unwedged.” The aortic valve between the atrioventricular valve annuli is anteriorly displaced (instead of being wedged). This elongates the left ventricular outflow tract (LVOT) in AVSDs.
Elongation of the Left Ventricular Outflow Tract
In normal hearts, the distance from the left ventricular (LV) apex to the aortic annulus is equal to the distance from the LV apex to the mitral annulus (Fig. 7.5). The inlet and outlet portions of the left ventricle are approximately equal in length. In contrast, the deficiency of the AV septum and apical displacement of the left AV valve insertion in AVSDs lends a scooped-out appearance to the ventricular septum and results in a shorter inlet portion. In addition, the anterior displacement of the unwedged aortic valve leads to an elongated and narrowed LVOT. The narrow, long LVOT has been classically described as having a “goose neck” appearance. This can be identified on angiographic and echocardiographic long-axis views of the left ventricle. This anatomic feature is clinically important since it provides the substrate for development of LVOT obstruction, especially when present with other findings such as aberrant left AV valve chordal insertions or displacement of a papillary muscle anteriorly into the LVOT.
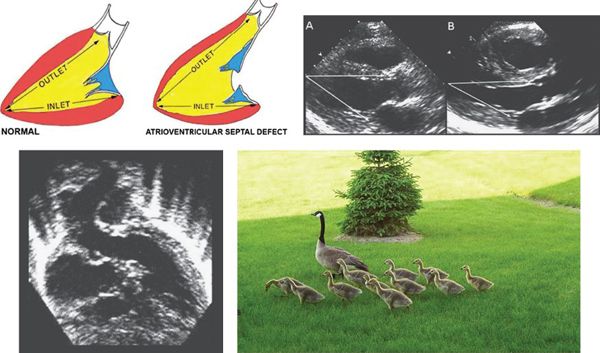
Figure 7.5. Left ventricular outflow elongation in atrioventricular septal defects (AVSD). The two diagrams in the upper left illustrate the LV inlet and outlet lengths in the normal heart (left) and in one with an AVSD (right). In patients with AVSD, the LV outlet is much longer than the inlet. Due to deficiency of the ventricular component of the atrioventricular septum and the “unwedged” aortic valve, the distance from the LV apex to the posterior left atrioventricular valve annulus is 20–25% shorter than the distance from the apex to the aortic annulus. Upper right: Pathologic specimens cut in long-axis projection in (A) a normal heart and (B) a heart with AVSD. These images demonstrate the changes in relative inlet/outlet length that were outlined by the diagrams. As a result, the distance from the LV apex to the aortic annulus is notably longer than the distance from the apex to the mitral annulus in AVSD, as opposed to nearly equal distances in the normal heart. Lower left: Apical five-chamber view demonstrating the elongated LVOT typical of AVSD. It has been described as a “goose neck.” Lower right: Springtime in Rochester, MN with pediatric and adult “goose necks.” (Upper left image with permission from Robert Anderson, MD.)
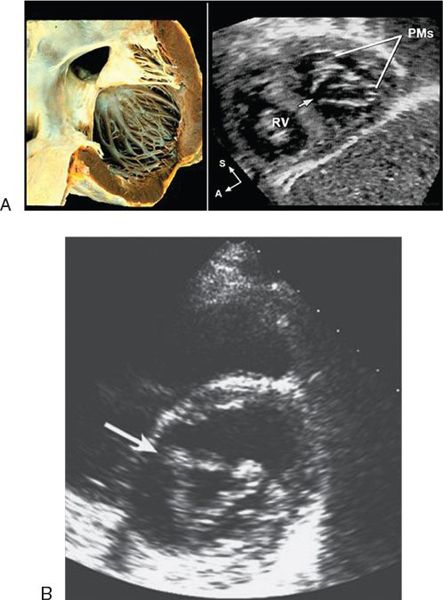
Figure 7.6. Cleft left atrioventricular valve. A: Left: In atrioventricular septal defect (AVSD), the cleft in the anterior leaflet of the left atrioventricular valve is typically oriented toward the mid-portion of the ventricular septum (arrow) along the anterior-inferior rim of the septal defect. Right: Subcostal sagittal image demonstrating the septal orientation of the cleft. B: The cleft (arrow) in the anterior leaflet gives the valve a trileaflet appearance as seen in this parasternal short-axis image.
Cleft of the Left Atrioventricular Valve
In partial AVSDs, the anterior mitral leaflet inserts onto the crest of the ventricular septum (Fig. 7.6). A cleft is invariably present in the anterior mitral leaflet and it is directed toward the mid-portion of the ventricular septum. In complete AVSDs, the common AV valve consists of five leaflets, and the two that span across the ventricular septum are known as the anterior and posterior bridging leaflets. Conceptually, the anterior bridging leaflet corresponds to the superior half of the anterior mitral leaflet, and the posterior bridging leaflet represents fusion of the septal tricuspid leaflet and the inferior portion of the anterior mitral leaflet. No tongue of tissue separates the AV valve into right and left components, and the space between the anterior and posterior bridging leaflets is analogous to the cleft in the anterior mitral leaflet in partial AVSDs.
Counterclockwise Rotation of the Left Ventricular Papillary Muscles
In all forms of AVSD, the LV papillary muscles are rotated counterclockwise compared with normal. In the parasternal short-axis projection, normal mitral papillary muscles are located at the “4 o’clock” and “8 o’clock” positions. In AVSD, LV papillary muscles are rotated toward the “3 o’clock” and “7 o’clock” positions. This causes the anterior mitral leaflet (or anterior bridging leaflet) to be more anteriorly located and contributes to narrowing of the LVOT.
ECHO EVALUATION OF AVSDS
Primum Atrial Septal Defects
Echocardiography is the diagnostic modality of choice for delineation of all anatomic features of AVSDs (Figs. 7.7 to 7.10). The best transducer position to define the number and size of ASDs is the subcostal view, as the plane of sound is perpendicular to the atrial septum. Both the subcostal four-chamber and sagittal (bicaval) views are helpful in that regard. Color Doppler delineates the shunt. The primum ASD in partial AVSD is typically large and easily visualized in the subcostal, parasternal, and apical four-chamber projections. The TEE four-chamber view readily demonstrates a primum ASD and the insertion of the tricuspid and mitral valves onto the crest of the septum.
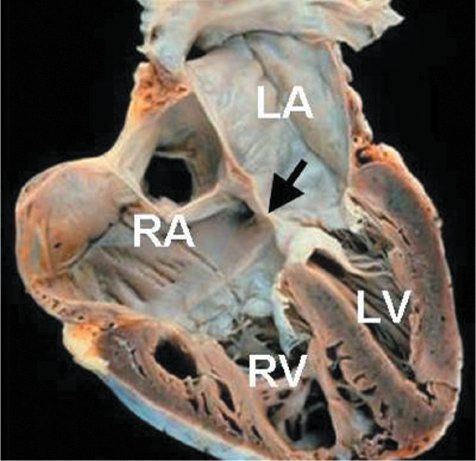
Figure 7.7. Partial atrioventricular septal defect (AVSD): Anatomic specimen. This heart has been cut in a plane simulating the apical four-chamber view and demonstrates the classic anatomy of a partial AVSD. The large primum atrial septal defect is highlighted by the arrow. Right atrial and right ventricular dilation are present. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
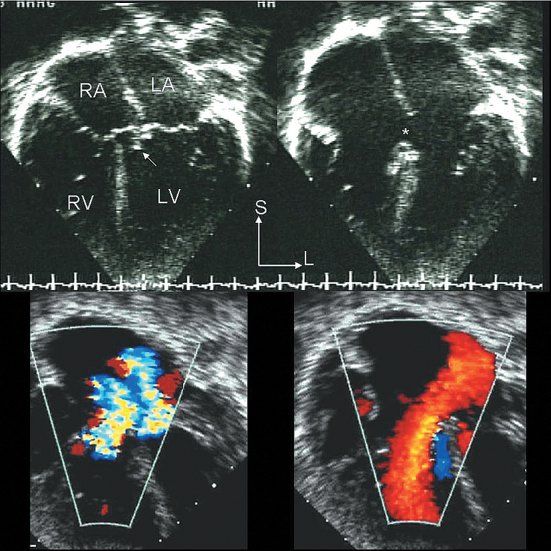
Figure 7.8. Partial atrioventricular septal defect (AVSD): Echocardiographic anatomy. Top/right: The four-chamber echocardiographic images in the two upper panels correspond to the anatomic plane shown in Figure 7.7. The upper left image was captured in systole and illustrates how the septal components of the two atrioventricular (AV) valves are at the same level. The potential VSD has been sealed by chordal attachments (arrow). The upper right panel was captured in diastole; it more clearly demonstrates the presence of a large primum ASD (asterisk) and the distinctly separate right and left AV valve orifices. The two color Doppler images in the bottom panels are paired with the two-dimensional images above them. The systolic frame on the left demonstrates regurgitation of both AV valves. The diastolic frame on the right shows a low velocity, but large, left atrial–to–right atrial/ventricular shunt crossing the ASD. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
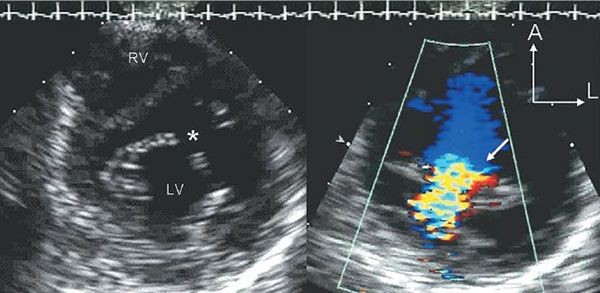
Figure 7.9. Cleft Mitral Valve associated with partial atrioventricular septal defect (AVSD). These two parasternal short-axis scans are focused on the anterior mitral valve leaflet in the left ventricular inflow tract. The image on the left is taken in diastole and demonstrates the division of the anterior leaflet into two separate components. This division creates a triangular (three-leaflet) appearance to the diastolic orifice of the mitral valve. The gap between the two components is referred to as the “cleft” (asterisk). The image on the right is a systolic color Doppler map demonstrating mitral regurgitation associated with the cleft. LV, left ventricle. RV, right ventricle.
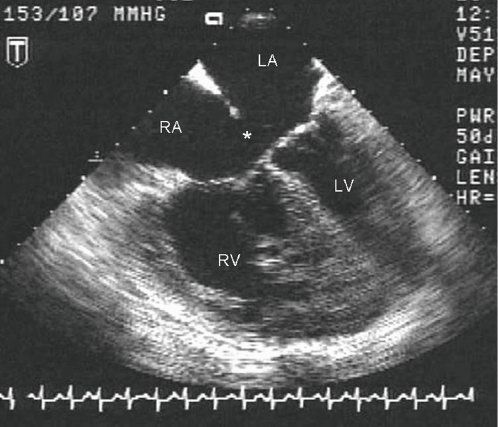
Figure 7.10. Partial atrioventricular septal defect (AVSD): Transesophageal Echocardiographic (TEE) anatomy. This diastolic four-chamber TEE image demonstrates a large primum ASD (asterisk) with insertion of both tricuspid and mitral valve leaflets to the crest of the ventricular septum. Marked RA and RV dilation are also evident, consistent with a large left-to-right shunt. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
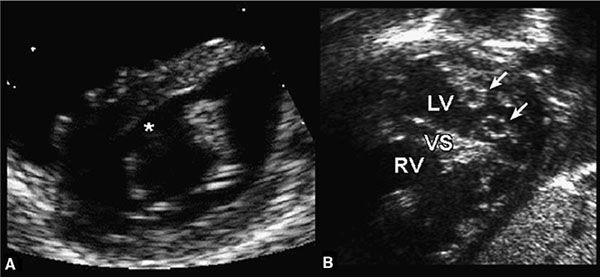
Figure 7.11. Cleft mitral valve (MV) and double-orifice atrioventricular valve. Left: Cleft MV. The anterior leaflet of the MV has a characteristic break (asterisk) that represents a cleft in the anterior leaflet. This feature is common to both partial and complete forms of atrioventricular septal defects (AVSDs). Right: Double-orifice atrioventricular valve. Each papillary muscle receives a separate atrioventricular valve orifice (arrows). The combined mitral orifice is less than the sum of both orifices and results in mitral stenosis. LV, left ventricle; RV, right ventricle; VS, ventricular septum. (Modified from Seward JB, Tajik AI, Edwards WD, et al. Two-Dimensional Echocardiographic Atlas. Vol. 1. Congenital Heart Disease. New York: Springer-Verlag, 1987:270–292.)
Mitral Valve Abnormalities
The cleft of the anterior mitral leaflet is best appreciated from subcostal and parasternal short-axis views (Fig. 7.11A
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


