ANATOMY
Atrioventricular (AV) canal defects have also been called endocardial cushion defects and AV septal defects (AVSDs). These defects are characterized by varying degrees of incomplete development of the septal tissue surrounding the AV valves along with varying degrees of abnormalities of the AV valves themselves. Consequently, AVSD may include defects in the inferior portion of the atrial septum, defects in the inflow portion of the ventricular septum, and defects in the tissue forming the left and right AV valves. We prefer the term AVSD because the anomaly is primarily caused by the deficiency of normal AV septal structures.
AVSDs represent a spectrum of cardiac anomalies subdivided into partial AVSDs, intermediate AVSDs, and complete AVSDs.
Partial AVSDs (also known as incomplete AVSDs) have a crescentshaped atrial septal defect (ASD) in the inferior portion of the atrial septum just above the AV valve. This defect may also be referred to as an ostium primum defect. The partial AVSDs also have varying degrees of malformation of the left AV valve, leading to varying degrees of left AV valve regurgitation.
Complete AVSDs have both defects in the atrial septum just above the AV valves and defects in the ventricular septum just below the AV valves. In complete AVSD, the AV valve is the one valve that bridges both the right and left sides of the heart, creating superior and inferior bridging leaflets. Partial AVSDs and complete AVSDs represent a spectrum of cardiac pathologic conditions. An intermediate form in the middle of this spectrum has been described and termed
intermediate AVSD (also known as transitional AVSD). This form of AVSD not only has two distinct left and right AV valve orifices but also has both an ASD just above and a ventricular septal defect (VSD) just below the AV valves. The VSD in this intermediate form of AVSD is often restrictive. Although these AV valves in the intermediate form do form two separate orifices, they remain as abnormal valves.
Table 86.1 provides definitions for AV canal defects provided by the International Society for Nomenclature of Paediatric and Congenital Heart Disease (www. ipccc.net).
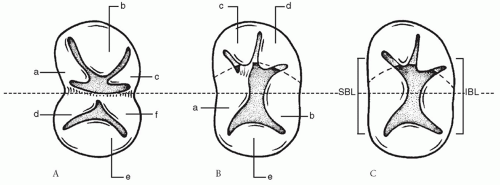
The AV valve apparatus in AVSD has been described as having either five or six leaflets. In partial AVSD (
Fig. 86.1A), the AV valve apparatus is easily understood as having six leaflets. On the left side, the leaflets have been termed left superior, left lateral, and left inferior. On the right side, the leaflets have similarly been termed right superior, right lateral, and right inferior. In partial AVSD, both the right superior and inferior leaflets fuse with the ventricular septum to complete the structure of the right AV valve. The left superior and inferior leaflets similarly fuse with the septum to form the left AV valve. The commissure between the left superior and inferior leaflets represents the “cleft” of the left AV valve, which is found in partial AVSD. This cleft is equivalent to the line of abutment (or zone of apposition) of the superior bridging leaflet and the inferior bridging leaflet in complete AVSD.
In complete AVSD (
Fig. 86.1B and 86.1C), it is more difficult to conceive of the common AV valve as a six-leaflet valve. A five-leaflet model is more realistic for complete AVSD. A superior bridging leaflet and an inferior bridging leaflet are always present. These bridging leaflets have very variable morphology in both the amount of leaflet that crosses over the ventricular septum and the degree of chordal attachment to the ventricular septum. Furthermore, scalloping of the bridging leaflets may create the illusion of extra leaflets. In addition to the superior and inferior bridging leaflets, the five-leaflet model is completed by a left lateral leaflet, a right lateral leaflet, and a right anterosuperior leaflet.
The degree of bridging and chordal attachment by the superior bridging leaflet forms the basis for the Rastelli classification of complete AVSD originally described in 1966. The Rastelli classification does not relate to the anatomy of the inferior bridging leaflet because this leaflet displays greater anatomic variation, and no consistent relationship exists between the morphology of the superior bridging leaflet and the inferior bridging leaflet. In a Rastelli type A defect (
Fig. 86.1B), the superior bridging leaflet is effectively limited to the left ventricle, with its right margin being attached to the ventricular crest. The anterosuperior leaflet of the right AV valve is also attached to the ventricular septal crest, giving the appearance that the superior bridging leaflet is split at the interventricular septum. In many cases, chordal attachments pull the plane of the AV valve down into the VSD below the plane of the annulus. In Rastelli type C defects (
Fig. 86.1C), there is a marked bridging of the ventricular septum by the superior bridging leaflet. The superior bridging leaflet floats freely over the ventricular septum without chordal attachment to the crest of the ventricular septum. Rastelli type B is somewhere between types A and C and is very rare in our experience; Rastelli type B involves anomalous papillary muscle attachment from the right side of the ventricular septum to the left side of the common superior (anterior) bridging leaflet.
The other important anatomic consideration when planning the repair of an AVSD is the location of the conduction system because it is very vulnerable during surgical repair (
Fig. 86.2). The AV node is displaced posteriorly and inferiorly toward the coronary sinus. The AV conduction axis then runs from this node toward the ventricle through the crest of the ventricular septum. Here, the posteriorly displaced bundle of His is usually covered by the inferior bridging leaflet of the AV valve. Thus, the AV node lies in the tissue between the coronary sinus and the margin of the VSD, if present. The location of the AV node is altered in AVSD because the ostium primum ASD often pushes the coronary sinus posteriorly and inferiorly toward the left atrium. This distorts the triangle of
Koch and creates a second triangle called the nodal triangle. The nodal triangle is bounded by the coronary sinus, the posterior attachment of the inferior bridging leaflet, and the leading edge of the atrial septum at the septal defect. The ASD pushes the AV node and the corresponding conduction tissues posteriorly and inferiorly along with the coronary sinus. The AV node, therefore, lies at the apex of the nodal triangle in a more posterior and inferior location. The bundle then travels down on the crest of the ventricular septum under the inferior bridging leaflet on the rim of the VSD.

SURGICAL TECHNIQUE
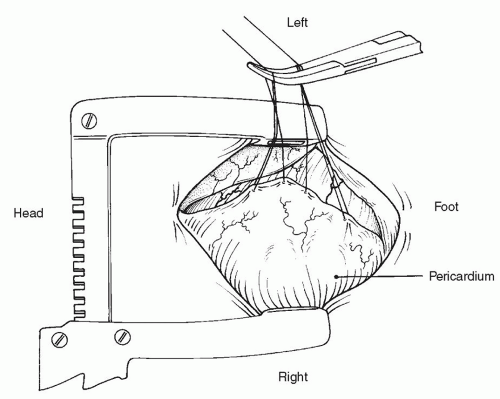
After routine anesthesia, preparation, and draping, a standard median sternotomy is performed followed by a thymectomy. An eccentric pericardiotomy to the left is then performed, leaving a large piece of pericardium attached on the right for later use as a patch (
Fig. 86.3). (All operative drawings presented here are viewed from the surgeon’s perspective.) The aorta, ductus arteriosus, and superior vena cava (SVC) are then mobilized. Identification of the ductus is best achieved by first finding the “axilla” between the right pulmonary artery and the ductus, and then finding the “axilla” between the left pulmonary artery and the ductus using traction on the main pulmonary artery. Once the right and left pulmonary arteries have been formally identified, anything remaining inbetween must be the ductus. A silk ligature is passed around the ductus but is not yet tied, with care being taken to avoid injuring the ductus.
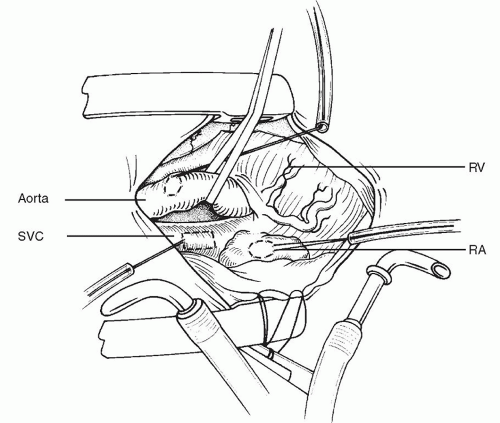
The lateral aspect of the SVC should be mobilized with scissors to avoid diathermy damage to the right phrenic nerve. Purse strings of 5-0 polypropylene are then placed into the aorta and the right atrial appendage. An additional, longitudinally oriented, narrow, rectangular purse string is placed on the SVC about 0.5 to 1.0 cm above the junction with the right atrium (
Fig. 86.4). The aorta is then cannulated. We prefer the DLP (Medtronic, Grand Rapids, MI) aortic cannula because of its flexibility. Next, the inferior vena cava (IVC) angled metal Pacifico venous cannula (DLP) is placed temporarily into the right atrial appendage. Cardiopulmonary bypass is then established (
Fig. 86.5), and the ductus arteriosus is ligated with the previously placed silk ligature. Once cardiopulmonary bypass is established, the patient is cooled. Some surgeons will cool to 24 or 25°C. Other surgeons do these cases at warmer temperatures such as 32°C. With warmer
temperatures, the hematocrit is kept higher while on cardiopulmonary bypass.
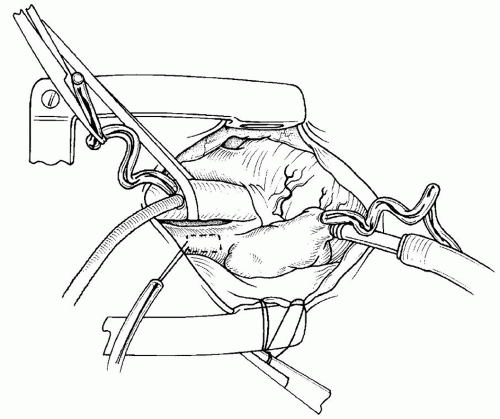
The SVC is then grasped with two mosquito hemostats on each side of the previously placed purse string. The SVC is incised longitudinally with a scalpel, and the SVC is then cannulated with the second angled metal cannula (
Fig. 86.6).
A vent is then inserted into the right atrial appendage after the IVC cannula is removed (
Fig. 86.7). The pericardial reflection anterior to the IVC is then released, and the sub-diaphragmatic IVC is exposed down to the level of the first hepatic vein. A purse string (5-0 polypropylene) is then placed directly into the IVC below the pericardial reflection (
Fig. 86.8), and the IVC is cannulated with the IVC angled metal cannula. Caval tapes are passed around the IVC and the SVC; nylon caval tapes may be used or silk suture may be used as caval tapes.
The aorta is cross-clamped, the two vena cavae are snared, the right atrium is opened parallel to the AV groove, and cardioplegic solution is instilled into the aortic root (
Fig. 86.9).
A right-angled instrument is then passed through the opening of the right atrium into the left atrium and up into the right superior pulmonary vein. A No. 11 scalpel blade is then used to open the junction of the right superior pulmonary vein and the left atrium between the tips of the right-angled instrument (
Fig. 86.10). The vent is then passed into the left atrium and secured into position with a 6-0 polypropylene purse-string suture.
Stay sutures are then applied to the atrial wall, and the anatomy of the AV valve is inspected (
Fig. 86.11). Stay sutures of 6-0 polypropylene on an 8-mm needle are then applied to approximate the “kissing points” of the bridging leaflets (
Fig. 86.12). The kissing points may be defined as the points of the superior bridging leaflet and the inferior bridging leaflet that (1) intuitively come together at the center of the superior bridging leaflet and the inferior bridging leaflets, (2) usually overlie the interventricular septum, and (3) can be identified as the midpoint between the left and right chordae on each bridging leaflet.
 ANATOMY
ANATOMY DIAGNOSIS
DIAGNOSIS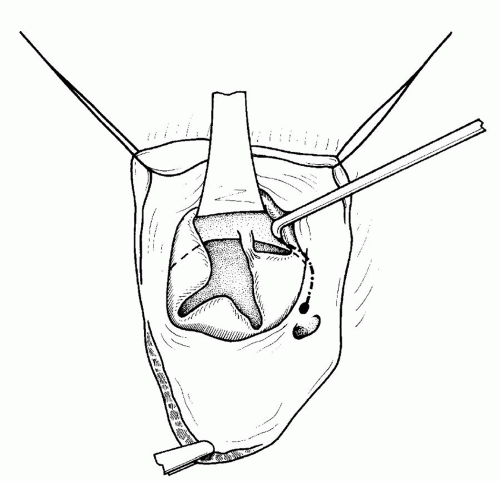
 INDICATIONS
INDICATIONS SURGICAL TECHNIQUE
SURGICAL TECHNIQUE