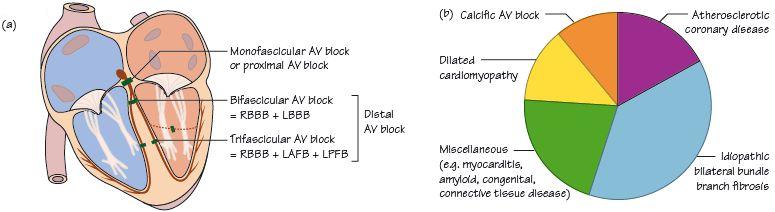
Fig. 57.2 (a) Site of block. (b) Causes of acquired complete heart block (CHB).
In third degree (complete) heart block (CHB) there is no electrical connection between the atria and the ventricle. It is common and serious – untreated, most patients with acquired CHB die within 2 months, many much earlier. At the moment when the electrical connection between the atria and the ventricle is severed, several events occur:
- Firstly, in many patients, ventricular standstill occurs, causing total loss of cardiac output and syncope. Ventricular asystole typically lasts for seconds rather than minutes. In some patients ventricular activity never resumes and death occurs.
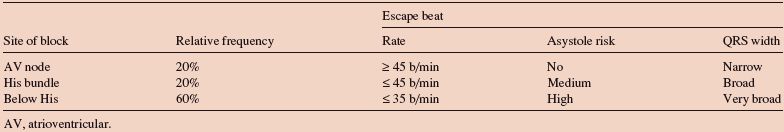
- Secondly, a spontaneous pacemaker starts up in the specialized conducting tissue of the ventricle immediately below the level of the block (Table 57.1). The higher up the block is in the conducting system, the higher the level of the tissue immediately below the block that can act as a pacemaker and the higher is the spontaneous heart rate (Fig. 57.1a,b). Spontaneous ventricular rates in CHB typically vary between 15–40 b/min. The higher the level of block, the more conducting tissue that the impulse can use, so the narrower the QRS complex.
ECG appearance of CHB
- The key ECG finding is atrioventricular (AV) dissociation, i.e. there is no relationship between the P waves and the QRS complex (Figs 57.1a,b & 57.2a,b).
- In atrial fibrillation (AF), P waves are not visible, so one cannot determine the relationship between atrial activity and QRS complexes. Complete heart block is diagnosed in AF by determining the regularity or otherwise of the QRS complexes. In AF with intact conducting tissue, QRSs occur irregularly. In AF with CHB, the QRS complexes occur regularly.
Symptoms in CHB
The symptoms are predictable from knowing the electrical events in CHB. With the onset of CHB, asystole develops and brief syncope results, followed by rapid and full recovery of higher mental faculties as the escape rhythm develops, restoring cardiac output. Though the heart is beating slowly, the cardiac output is rarely depressed enough to cause symptoms at rest. However, the exercise heart rate and cardiac output are greatly depressed, resulting in effort breathlessness, fatigue, possibly angina, and tiredness. Examination shows bradycardia, AV dissociation in the venous pressure and variable intensity of the first heart sound, an ejection flow murmur and sometimes signs of heart failure.
Prognosis
The outlook in the absence of treatment is poor. Increasingly long episodes of asystole followed by increasingly slow escape beats are likely, resulting in further syncope, until finally asystole occurs not followed by a ventricular escape rhythm and death. Occasionally the bradycardia leads to a high-grade ventricular arrhythmia, often a long QT dependent arrhythmia such as torsade-de-pointes. Clearly this is lethal unless terminated quickly.
Treatment
Most acquired CHB requires a permanent pacemaker. Whether a temporary pacemaker (‘wire’) is needed prior to the permanent pacemaker depends on the stability of the escape rhythm, the safety of temporary wire insertion locally and the delay in permanent pacemaker implantation. Unstable rhythms are those where syncope (i.e. significant asystole) has occurred, where the heart rate is very low (i.e. ≤ 30 b/min) or the QRS complex is broad. Escape beats with a continually changing QRS morphology indicate a very high risk of early asystole. Complete heart block that does not require a temporary or permanent pacemaker needs to have a stable escape rhythm and a reversible cause, e.g. CHB complicating an inferior wall ST segment elevation myocardial infarction (STEMI). Congenital CHB (due to maternal systemic lupus erythematosus [SLE] damaging fetal cardiac conducting tissue in utero) is often managed without pacing, though there is accumulating data that permanent pacemakers may prolong life.
Table 57.1 Relative frequency, clinical characteristics and ECG findings in third degree AV block due to conduction block at different sites.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


