Introduction
Atrial fibrillation (AF) has been described as an “epidemic” due to its rising prevalence in our aging population. AF is a leading cause of thromboembolism and is also asscociated with impaired quality of life and increased overall mortality. Available treatment strategies consist of heart rate control, rhythm control and prevention of thromboembolism. Antiarrhythmic agents still form the mainstay of pharmacologic therapy for AF. However, despite their proven efficacy in symptom control and prevention of hemodynamic complications, they do not offer a definitive treatment. Comprehensive understanding of the mechanisms leading to AF is paramount for the development of targeted and potentially curative treatments.
Current evidence suggests that the pathogenesis of AF is multifactorial. Observations at the level of atrial cardiomyocytes and interstitium reveal cellular degeneration with hypertrophy and extracellular matrix protein proliferation leading to fibrosis. It has been described that interstitial fibrosis predisposes to tissue anisotropy by creating areas with different conduction properties, which can either block or initiate a conduction wavelet and lead to re-entry circuits and AF. Although this structural remodeling can generate an inflammatory response, inflammation may also play a direct role in the development of atrial fibrosis and act as a trigger for the development of AF in the presence of a susceptible anatomic substrate. Finally, the ionic current changes and action potential abnormalities associated with the development of AF are also accountable for the self-perpetuation of the arrhythmia.
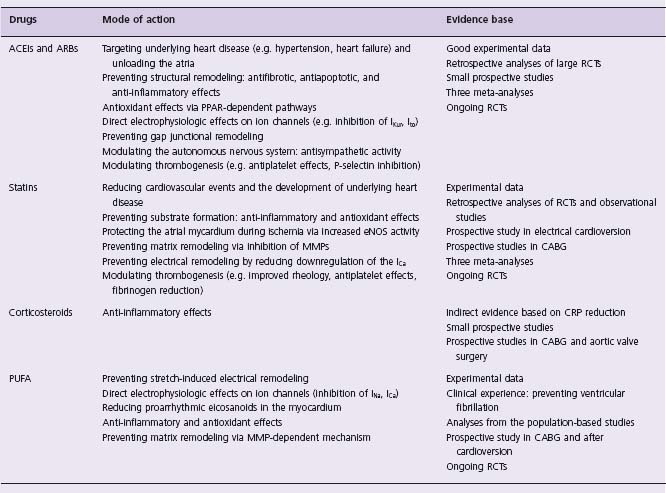
Upstream management of AF refers to therapies that target the responsible substrate and aim to prevent the development of the arrhythmia in the first place or delay its occurrence and domestication. Experimental and clinical data have highlighted the potential effect of angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), statins, n-3 polyunsaturated fatty acids (PUFA) and corticosteroids on the prevention of AF. The proposed mechanisms by which these agents exhibit their protective effect are shown in Table 36.1.
In a meta-analysis of 12 published randomized controlled trials (RCT) evaluating the effect of ACEIs/ARBs on new or recurrent AF, the difference in the underlying cardiovascular pathology of the studied populations led to significant variability in outcomes.1 Although ACEIs/ARBs conferred a statistically significant risk reduction of 28% in AF, this effect was more pronounced in high-risk heart failure patients (44% relative risk reduction), and absent in the hypertension subgroup analysis. This finding was consistent even when the meta-analysis was revisited by another group, which included data from patients with cardiovascular disease but preserved left ventricular ejection fraction (LVEF), receiving ramipril or placebo.2 In another meta-analysis,3 additional to their marked benefit in patients with heart failure, ACEIs/ARBs were also found to be effective in the prevention of AF in patients with hypertension and following myocardial infarction (23% and 11% risk reduction, respectively). Finally, Anand and colleagues, having identified 9 RCTs with new onset AF (and not recurrent) as a reported outcome, demonstrated that ACEIs were more efficacious in primary AF prevention than ARBs.4 Overall, most of the studies examined did not specify AF as a primary endpoint and AF outcomes were analysed post hoc, which may have led to multiple testing errors. This evidence-based assessment of the efficacy of ACEIs/ARBs in AF prevention focuses on patient groups with similar underlying cardiovascular pathology.
ACEIs and ARBs for the prevention of AF in patients with heart failure
The beneficial effects of ACEI/ARB therapy in patients with heart failure are well established. Their potential antiarrhythmic effect was initially examined in a cohort of 30 patients with congestive heart failure (CHF) and persistent AF who were randomized to lisinopril 10 mg or placebo and treated with electrical cardioversion.5 Despite the higher rate of maintenance of sinus rhythm (SR) in the lisinopril group, the AF risk reduction was not statistically significant in this small group of patients.
The first study to demonstrate a statistically significant reduction in AF due to ACEI treatment was the TRAndolapril Cardiac Evaluation (TRACE) trial.6 Patients admitted to hospital with acute MI and documented LVEF ≤36% were randomized to trandolapril (up to 4 mg/day) or placebo. During the follow-up period of 2–4 years, the incidence of AF was 5.3% in the plecebo group and 2.8% in the treatment group, yielding a 55% risk reduction in AF due to trandolapril. Although the antiarrhythmic effect of trandolapril remained consistent after adjustment for progressive LVEF decline in the placebo group, it is likely that AF reduction may just reflect the distinct attenuation of LV remodeling following MI due to ACE inhibition. The results of the SOLVD (Studies Of Left Ventricular Dysfunction) trial, which examined the effect of ACE inhibition on AF in patients with LV dysfunction, supported the beneficial role of enalapril.7 With an absolute risk reduction of 18%, enalapril use was strongly associated with maintenance of SR during the 2.9 years of follow-up. Once again, it is likely that the efficacious management of underlying cardiac disease might have been responsible for this reduction in AF rather than any potential direct antiarrhythmic effect of enalapril.
In 4395 patients with documented heart failure who were randomized to valsartan or placebo in the context of the Val-HeFT study,8 new-onset AF occurred in 5.1% of those on valsartan versus 7.95% receiving placebo (odds ratio (OR) 0.63, 95% confidence interval (CI) 0.49–0.81). This risk reduction in AF due to valsartan was not influenced by concomitant treatment with other ACE inhibitors (93% of cases). In addition, AF occurrence was associated with a 40% increase in overall mortality. Despite the efficacy of valsartan in the prevention of AF, its capacity to reduce mortality was not proven, probably due to the relatively low rate of AF in this study population. In the CHARM study,9 the effect of candesartan on new-onset AF was a prespecified secondary outcome in the cohort of 6379 patients with symptomatic heart failure and absence of the arrhythmia. The reduction in AF caused by candesartan (target dose 32 mg/d) versus placebo (5.55% vs 6.74%), remained significant following adjustment for prespecified co-variates that are known to influence overall prognosis and AF development (OR 0.80, 95% CI 0.65–0.99). The design of this study allowed for the analysis of subgroups with CHF but preserved LVEF (in contrast to the SOLVD and Val-HeFT studies), and CHF with or without concomitant ACEI treatment. Although no significant heterogeneity was observed in subgroup analysis, the beneficial effect of candesartan was more pronounced in patients with reduced LVEF, irrespective of ACEI treatment (adjusted OR 0.77, 95% CI 0.59 –0.99).
Table 36.2 Clinical studies of ACEIs and ARBs for the prevention of atrial fibrillation
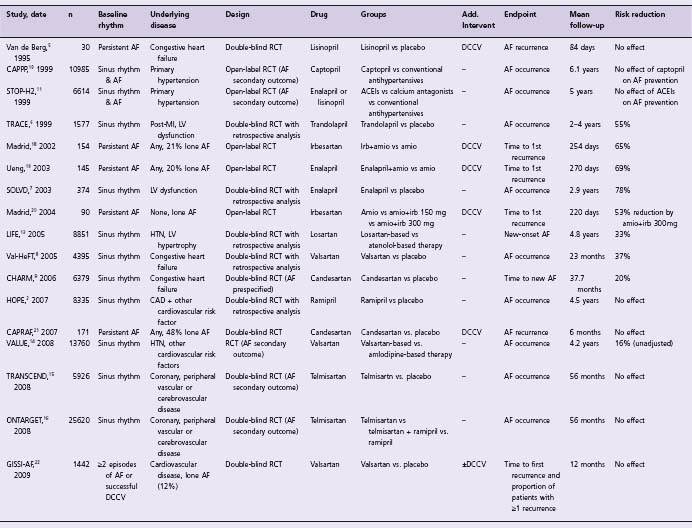
The available data from these large-scale studies are highly suggestive of the preventive role of ACEIs/ARBs in avoiding new or recurrent AF in patients with heart failure. Although this association was often either a post hoc finding or a secondary endpoint, the size of the studies and the consistency of their results provide satisfactory evidence on the efficacy of ACEIs/ARBs in this setting. Therefore, the use of ACEIs/ARBs for the prevention of AF in patients with CHF is recommended (Class I, Level A).
ACEIs and ARBs for the prevention of AF in patients with hypertension and/or coronary artery disease
The efficacy of ACEIs/ARBs for the treatment of primary hypertension is recognized. Large studies, which aimed to identify whether their antihypertensive capacity is associated with a reduction in overall cardiovascular mortality and morbidity, included AF occurrence in their secondary endpoints. More specifically, the Captopril Prevention Project (CAPPP) was a multicenter randomized trial evaluating the effect of captopril (50–100 mg) versus conventional antihypertensive treatment with beta-blockers and/or diuretics, on fatal and non-fatal myocardial infarction and stroke and other cardiovascular deaths.10 Cardiovascular mortality was lower in the captopril group, albeit not statistically significant. No difference was observed upon analyses of secondary outcomes with the inclusion of new AF, between the two groups. These findings were in line with its contemporary, the Swedish Trial in Old Patients with Hypertension-2 (STOP-H2) study,11 which compared the efficacy of conventional antihypertensives (diuretics, beta-blockers), ACEIs (enalapril 10 mg, lisinopril 10 mg) and calcium antagonists (felodipine 2.5 mg, isradipine 2.5 mg) on cardiovascular morbidity and mortality in patients aged 70–84 years. All three therapies were similarly effective against these primary outcomes, and no superiority of ACEIs was observed in the prevention of AF when compared to conventional antihypertensives. However, in another multicenter trial (LIFE),12 patients with hypertension and documented LV hypertrophy benefited from losartan-based treatments, which significantly reduced new-onset AF when compared to atenolol-based treatment (OR 0.67, 95% CI 0.55–0.83). The enhanced regression of LV hypertrophy caused by losartan, as evaluated on the echocardiographic substudy within LIFE,13 may have led to reduced atrial overload and dilation, and therefore a reduction in AF. New-onset AF was a prespecified outcome within the VALUE trial.14 Valsartan-based antihypertensive treatment led to a reduction in AF occurrence when compared to amlodipine in patients with hypertension and other cardiovascular risk factors. In contrast, two contemporary RCTs evaluating the role of telmisartan versus placebo,15 and telmisartan with or without ramipril,16 did not show any preventative effect on AF occurrence in patients with coronary, peripheral or cerebrovascular disease.
In conclusion, due to the limited number of studies, their design and significant heterogeneity, the efficacy of ACEIs/ ARBs in the prevention of AF in hypertensive patients with cardiovascular risk factors, although plausible, requires further evaluation (Class IIa, Level B).
ACEIs and ARBs for secondary prevention of AF
The efficacy of amiodarone in the maintenance of sinus rhythm in patients with persistent AF is well documented.17 The addition of irbesartan (150–300mg/d) to amiodarone (400 mg) therapy before electrical cardioversion for persistent AF led to a 65% reduction in the risk of AF recurrence over a two-month follow-up.18 This open-label randomized trial was the first to describe the possible association of ARBs and maintenance of sinus rhythm. However, it was limited by the fact that the irbesartan treatment group was more likely to receive beta-blockade, which is known to be protective for AF, and that asymptomatic recurrences that terminated spontaneously could not have been effectively captured. In a similar study evaluating the effect of enalapril on AF recurrence following electrical cardioversion and amiodarone, a 69% risk reduction was observed in patients on combined enalapril and amiodarone treatment at four weeks.19 Finally, Madrid and colleagues20 examined the effect of increasing doses of irbesartan in addition to amiodarone treatment on AF recurrence after electrical cardioversion in patients with “lone” persistent AF. Maintenance of SR was higher in the irbesartan 300 mg plus amiodarone group (77%), when compared to the irbesartan 150 mg plus amiodarone group (65%) and to amiodarone alone (52%). The risk reduction conferred by high-dose irbesartan was 53% after appropriate adjustments at the two-month follow-up.
Although a combination therapy of amiodarone with ACEIs/ARBs appears superior to amiodarone alone, the efficacy of monotherapy with ACEIs/ARBs was not tested in any of those trials. In addition, their open-label design and the lack of placebo group may have influenced the accuracy of their outcomes. In an attempt to overcome these important limitations, Tveit and colleagues designed a double-blind placebo-controlled trial examining the effect of the ARB candesartan on AF recurrence following successful electrical cardioversion in patients not receiving adjunct antiarrhythmics.21 Six-week pretreatment with can-desartan (16 mg) followed by a 6-month therapy after electrical cardioversion did not influence AF recurrence rates when compared with placebo. Similarly, the more recent GISSI-AF study, which examined the role of valsartan (vs. placebo) in AF prevention in patients with documented symptomatic AF and/or successful electrical cardioversion, did not demonstrate any effect attributed to valsartan treatment.22 The main outcome measures were time to first AF recurrence and proportion of patients with more than one AF episodes during the first year; the treatment arm received valsartan gradually adjusted to 320 mg daily, in addition to established antiarrhythmic regimens. Despite the relatively short follow-up and the underrepresentation of patients with heart failure (8%), the negative results from this study encourage the scepticism regarding the efficacy of ARBs in secondary AF prevention.
Therefore, the beneficial effect of ACEIs/ARBs in secondary prevention is not yet established and will need to be validated further (Class IIb, Level B). Ongoing trials aim to create a clearer picture on the antiarrhythmic potential of ACEIs/ARBs. ACTIVE I is a partial factorial placebocontrolled, double blind trial evaluating the capacity of irbesartan (titrated to 300 mg/day) to prevent AF recurrence in patients with paroxysmal or persistent AF, which is a prespecified secondary outcome.23 The Angiotensin II antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial focuses on the effect of olmesartan (40 mg/day) on reduction of episodes of paroxysmal AF as a primary endpoint, and assesses both symptomatic and asymptomatic paroxysms with the use of transtelephonic ECG monitoring.24
Statins for the prevention and treatment of atrial fibrillation
Several studies have suggested the possible prophylactic effect of statins on new-onset AF and on AF recurrence following successful electrical or chemical cardioversion.
A systematic review and meta-analysis evaluating the efficacy of statins on AF prevention demonstrated variability in outcomes between observational studies and RCTs.25 Interestingly, the antiarrhythmic effect of statins was less pronounced in RCTs and did not reach statistical significance, whereas observational studies yielded a 23% relative risk reduction in patients on statins, which was statistically significant (OR 0.77, 95% CI 0.70–0.85) and associated with no apparent heterogeneity. Many reasons may account for this discrepancy, such as the potentially underpowered AF detection in a large RCT, longer mean follow-up of observational studies and variability in methods of AF detection. It is also evident that the patient populations, presence and type of AF at baseline, type of intervention performed, and primary outcome of interest differed between studies. Another meta-analysis focusing only on RCTs demonstrated that statin use was associated with a significant decrease in the incidence or recurrence of AF (OR 0.39, 95% CI 0.10–0.85). However, the variability in clinical settings and significant heterogeneity between studies is, once again, supportive but not confirmatory of the overall beneficial effect of statins on AF.26 This is why our evidence-based analysis examines the effect of statins on patients according to their baseline heart rhythm, underlying cardiovascular pathology and type of intervention performed.
Statins for the prevention of AF in patients with coronary artery disease
The first association of statin use with prevention of AF in patients with chronic coronary artery disease (CAD) was shown by Young-Xu and colleagues in an observational longitudinal study with a five-year follow-up.27 Patients receiving statins had a 9% incidence of new-onset AF compared with 15% in patients not on statins. Following adjustment for potential confounders, the risk reduction in AF remained statistically significant (OR 0.37, 95% CI 0.18–0.76). This effect was independent of changes in baseline and follow-up cholesterol levels, indicating that the potential antiarrhythmic effect of statins extends beyond their lipid-lowering capacity.
High-dose statin use and its possible impact on AF in patients with acute coronary syndrome (ACS) was examined post hoc within the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) study.28 Atorvastatin 80 mg or placebo was administered to patients with ACS within four days of admission to hospital and for a period of 16 weeks. The incidence of new-onset AF (1.6% and 1.8%, respectively) in patients without the arrhythmia prior to randomization and the rate of freedom from recurrence in patients with known AF, were not influenced by intensive statin treatment. However, the short duration of follow-up (16 weeks) with limited ECG recordings may be responsible for the very low incidence of new-onset AF, which potentially renders this analysis underpowered. In a retrospective study,29 1526 patients admitted to hospital with suspected ACS and no history of arrhythmia were evaluated by the development of new AF and statin use. A risk reduction of 43% was present in statin users following adjustment for potential confounders (OR 0.57, 95% CI 0.39–0.83).
Table 36.3 Clinical studies of statins for the prevention of atrial fibrillation
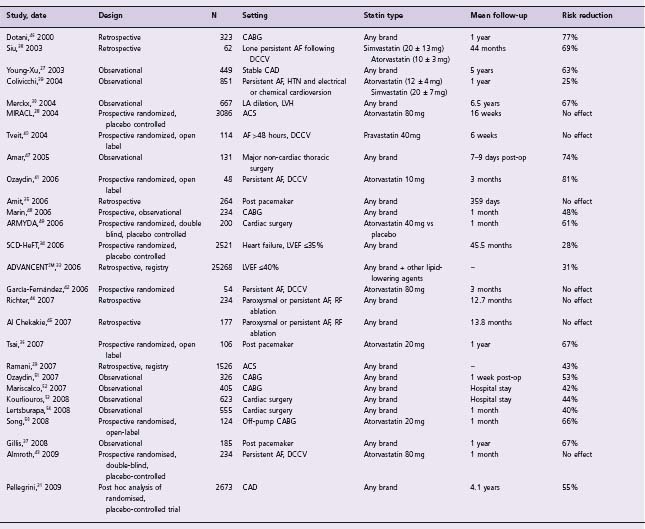
Echocardiographic risk factors for the development of AF, such as left ventricular hypertrophy (LVH) (wall thickness ≥10mm) and left atrial dilation (≥40mm) were evaluated in another study by Merckx and colleagues.30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree