Introduction
The Framingham Study in the US and the Rotterdam Study in Europe have estimated lifetime risk for development of atrial fibrillation (AF) to be 1 in 4 for men and women 40 years of age and older.1,2 Projected data from two population-based studies in the US predict a 2.5 to 3-fold increase in the number of patients with AF by 2050.3,4 The high lifetime risk for AF and increased longevity underscore the important public health burden posed by this arrhythmia across the world. Because AF has multiple etiologies and a broad variety of presentations it demands a range of therapeutic responses. The primary pathologies underlying or promoting the occurrence of AF vary more than for any other cardiac arrhythmia, ranging from autonomic imbalance through organic heart disease to metabolic disorders, such as diabetes mellitus, metabolic syndrome, and hyperthy-roidism. A rational approach to management of the individual case depends on careful assessment of the temporal pattern of the arrhythmia, associated cardiovascular disease, and any particular features suggesting the advisability or risks of any particular treatment regimen. The nature of AF and of individual patient factors change over time, requiring a flexible approach to long-term management.
AF usually tends to recur and may follow a predictable pattern. Paroxysmal AF generally progresses to persistent and/or permanent over time. In the Canadian Registry of AF (CARAF) 8–9% of patients with new-onset paroxysmal AF developed a sustained arrhythmia by the end of the first year; about 25% were in permanent AF by 5 years.5 Rates appear to be higher for those with a persistent variety, with 35–40% developing the permanent arrhythmia by the end of the first year since diagnosis.6,7 Between two-thirds and three-quarters of patients had documented recurrence of AF within 5 years, despite continuous antiarrhythmic drug therapy.5 However, even these projections may represent conservative estimates because of undiscovered silent arrhythmias.8 Progression of AF relates to progression of the underlying disease and to continuous structural remodeling of the atria, including changes associated with ageing (e.g. fatty metamorphosis, myocyte degeneration, and fibrosis). As the arrhythmia becomes more sustained, restoration and maintenance of sinus rhythm become more challenging.
The fundamental principles of therapy for AF include primary prevention (i.e. treating the conditions that are commonly associated with the development of the arrhythmia); cardioversion of first-onset or recurrent AF which fails to self-terminate; secondary prevention (i.e. specific antiarrhythmic drugs or catheter ablation to suppress recurrence of paroxysmal or persistent AF once the arrhythmia has occurred); control of ventricular rates during recurrent or permanent AF; and anticoagulation to prevent thromboembolism. Treatment of underlying heart disease, such as hypertension and heart failure may per se deter the occurrence of new AF by alleviating and/or delaying remodeling of the atria (primary prevention). Although secondary prevention usually implies the use of specific antiarrhythmic agents to prevent recurrent AF, continuous treatment of underlying heart disease (e.g. stringent blood pressure control in hypertension) is likely to contribute to the reduction of recurrence and also to delay the progression to permanent AF. Treatment of the underlying heart disease to prevent AF is often referred as “upstream” therapy.9 There is evidence that beta-blockers, which have very modest antiarrhythmic properties, and traditionally non-antiarrhythmic drugs, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and statins, may have the antiarrhythmic potential, additional to any treatment effect on the underlying disease. This is discussed in Chapter 36.
Non-pharmacologic rhythm control therapy with catheter-based pulmonary vein isolation and left atrial ablation can be used to treat some forms of AF. However, the eventual impact of catheter-based techniques, particularly for long-term rhythm control and in patients with significant underlying heart disease, is not yet known.10 Drug therapy remains, therefore, the mainstream for management of AF. Rate control is the primary treatment strategy in permanent AF and it is also relevant to all forms of the arrhythmia.
Studies of rate and rhythm control strategies
Two prime treatment strategies are rhythm control and rate control. Intuitively, rhythm control is a more attractive treatment strategy as sinus rhythm offers physiologic control of heart rate and regularity, normal atrial activation and contraction, the correct sequence of atrioventricular activation, and normal valve function. It might be an ideal approach for both stroke prevention and symptom alleviation. Nevertheless, during the 1980s and 1990s, the potential downside of available antiarrhythmic drug therapy became clear. A series of studies showed that under certain conditions, antiarrhythmic drugs could become proarrhythmic and, thereby, potentially lethal.11,12 And for amiodarone, although proarrhythmia uniquely was an infrequent clinical problem, pulmonary fibrosis, a potentially lethal adverse effect, could develop.13
The difficulties in rhythm control management, principally the high AF recurrence rate, and the concern for serious adverse effects associated with antiarrhythmic drug therapy finally led to rate versus rhythm control studies. The major studies were the Atrial Fibrillation Follow up Investigation of Rhythm Management (AFFIRM) trial14, the RAte Control versus Electrical Cardioversion (RACE) trial15 and, most recently, the Atrial Fibrillation Congestive Heart Failure (AF CHF) trial16 (Table 35.1). There were also a series of pilot studies performed, including the Pharmacological Intervention in Atrial Fibrillation (PIAF),17 Strategies of Treatment of Atrial Fibrillation (STAF),18 and How to Treat Chronic Atrial Fibrillation (HOT CAFÉ),19 among others. They all directly and prospectively compared the effect of rhythm control treatment strategies versus rate control strategies on patient outcomes. It should be emphasized that it is likely none of these studies would have been performed if an antiarrhythmic drug or drugs were available which suppressed AF with >90% efficacy and an acceptable adverse effect profile.
Table 35.1 Studies of rate versus rhythm control in atrial fibrillation
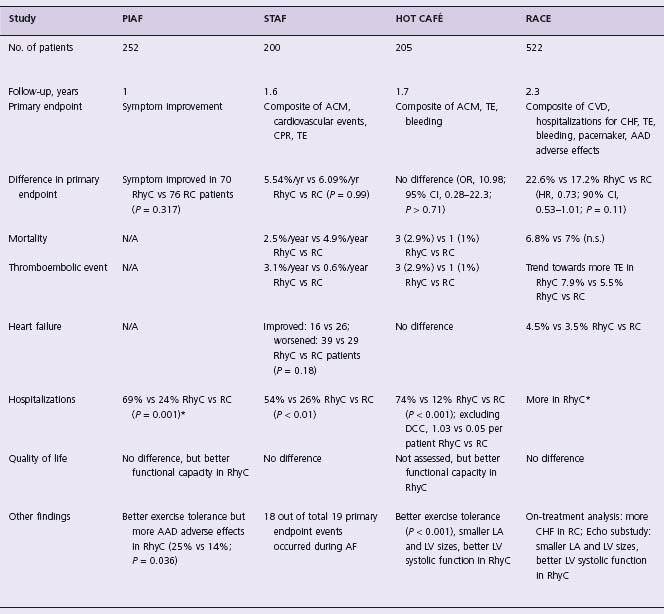
AAD, antiarrhythmic drugs; ACM, all-cause mortality; AF, atrial fibrillation; AF CHF, Atrial Fibrillation and Congestive Heart Failure; AFFIRM, Atrial Fibrillation Follow-up Investigation of Rhythm Management; CHF, congestive heart failure; CI, confidence intervals; CPR, cardiopulmonary resuscitation; CRRAFT, Control of Rate versus Rhythm in rheumatic study in rheumatic Atrial Fibrillation Trial; CVD, cardiovascular death; HOT CAFÉ, HOw to Treat Chronic Atrial Fibrillation; HR, hazard ratio; J-RHYTHM, Japanese Rhythm management trial for atrial fibrillation; LA, left atrium; LV, left ventricle; OR, odds ratio; PAF, Paroxysmal Atrial Fibrillation 2; PIAF, Pharmacological Intervention in Atrial Fibrillation; RACE, Rate Control versus Electrical Cardioversion; RC, rate control; RhyC, rhythm control; SE, systemic embolism; STAF, Strategies of Treatment of Atrial Fibrillation; TE, thromboembolic event; TIA, transient ischemic attack. *, including cardioversion;†, reported as an adverse event
AFFIRM
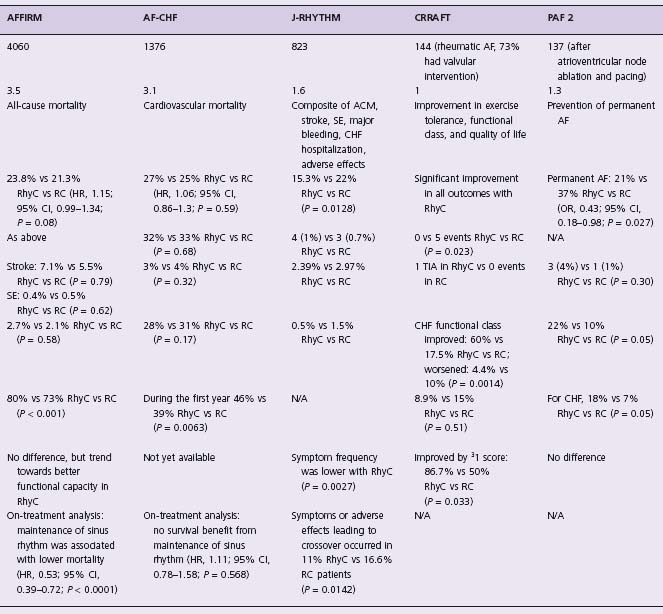
AFFIRM was a randomized clinical trial that compared two treatment strategies for AF in 4060 patients with paroxysmal or persistent AF and one or more risk factors (age ≥65, hypertension, diabetes, poor left ventricular function, congestive heart failure, or a prior stroke or transient ischemic attack) associated with a high risk of stroke and death, with at least 6 hours of AF in the last 6 months (with one episode in the previous 12 weeks).14 The primary endpoint was all-cause mortality. A combined secondary endpoint consisted of death, disabling stroke or anoxic encephalopathy, major bleed or cardiac arrest. Quality of life and functional capacity were also assessed in a subset of patients. Throughout the study, the prevalence of sinus rhythm was high in the rhythm control arm, although it declined with time (82.4% at 1 year, 73.3% at 3 years, and 62.6% at 5 years). The prevalence of sinus rhythm in the rate control arm was higher than anticipated (42.9% at 1 year, 38.5% at 3 years, and 34.6% at 5 years).
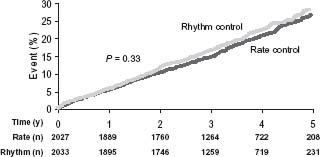
A total of 356 patients in the rhythm control arm and 306 patients in the rate control arm died during this study. Overall survival at 1, 3, and 5 years was 96%, 87%, and 76% in the rhythm control arm, and 96%, 89%, and 79% in the rate control arm (Fig. 35.1).14 Although there was no significant difference between the two groups (P = 0.08), a trend towards better survival in the rate control arm appeared after the first 1½ years. The secondary combined endpoint (death, disabling stroke or anoxic encephalopathy, major bleed or cardiac arrest) was not significantly different between the arms (P = 0.33) (Fig. 35.2).20 No major differences were noted in functional status or quality of life.
Figure 35.1 Cumulative mortality from any cause in the rate control and rhythm control arms of the AFFIRM study. Time zero is the day of randomization. Data have been truncated at 5 years. (From Wyse et al.14, with permission.)
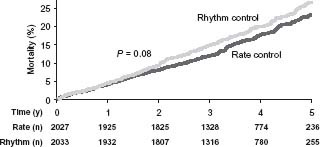
Figure 35.2 Cumulative events for the secondary endpoint of death, disabling stroke or anoxic encephalopathy, major bleed or cardiac arrest in the AFFIRM trial. Time zero is the day of randomization. Data have been truncated at 5 years. (From Waldo20, with permission.)
A total of 77 ischemic strokes occurred in the rate control arm compared with 80 in the rhythm control arm (P = 0.79).14 Most strokes in both arms occurred in patients who were either not taking warfarin or who had an INR < 2.0. In the rhythm control arm, 22% of strokes occurred in patients whose INR was < 2, and more than one-half (57%) occurred in patients not taking warfarin. Thus, 79% of patients in the rhythm control arm who had a stroke either were not taking warfarin or the INR was subtherapeutic. These stroke outcomes probably should not be surprising in patients with stroke risk factors because of the likely recurrence of AF in a large percentage of patients and the fact that there is an important incidence of asymptomatic AF.
RACE
RACE was a multicenter, randomized trial in 522 patients.15 It tested the hypothesis that rate control is not inferior to the maintenance of sinus rhythm for the treatment of persistent AF. The primary endpoint was a composite of cardiovascular death, hospital admission for heart failure, thromboembolic complications, severe bleeding, pacemaker implantation, and severe adverse effects of therapy. Rate control was achieved by keeping the ventricular response rates < 100 beats per minute with control of symptoms. Rhythm control consisted of serial use of antiarrhythmic drug therapy and electrical cardioversion, if required, to maintain sinus rhythm.
At 3-year follow-up, only 40% of rhythm control patients and 10% of rate control patients were in sinus rhythm. In this trial, 17.2% of patients achieved the primary endpoint in the rate control arm, versus 22.6% of patients in the rhythm control arm (absolute difference 5.4%; 90% confidence interval (CI)-11% to 0.4%). Therefore, the RACE trial demonstrated that rate control met the criteria for non-inferiority to rhythm control, indicating that rate control is an acceptable primary treatment option for patients with AF.
PIAF
The PIAF pilot study17 compared rate control using diltiazem with rhythm control using amiodarone. Other atrio-ventricular node blocking drugs could be used if diltiazem failed to control the ventricular rate. The trial enrolled 252 patients with persistent AF. The primary endpoint was improvement in AF-related symptoms. At follow-up, 56% of patients in the rhythm control arm and 10% of patients in the rate control arm were in sinus rhythm (P < 0.001). At 1 year, a similar proportion of patients in both groups reported improved symptoms (76 patients in the rate control arm vs 70 patients in the rhythm control arm, P = 0.317). Amiodarone restored sinus rhythm in 23% of patients. The distance walked in 6 minutes was greater in the rhythm control arm, but quality of life measures did not differ. Hospital admission rates were higher in the rhythm control arm (87/127 (69%) vs 30/125 (24%), P = 0.001), and adverse drug effects caused more frequent therapy changes in this arm as well (31 (25%) vs 17 (14%), P = 0.036). Overall, although the two groups in the PIAF study had similar clinical results, exercise tolerance was better with rhythm control. However, this advantage in favor of rhythm control was offset by more frequent hospital admissions.
STAF
In this pilot study, 200 patients with persistent AF were randomized to rate control or rhythm control, and followed for a mean of 19.6 ± 8.9 months.18 There was no difference between the two strategies for the primary combined end-point of death, cardiopulmonary resuscitation, stroke or transient ischemic attack, and systemic embolism. Hospitalization was significantly more frequent in the rhythm control arm, explained by the need to perform multiple cardioversions and to change antiarrhythmic drug therapy. In short, the data showed similar outcomes in both arms, supporting the concept that rate control is a legitimate primary treatment option. Moreover, the study suggested there was no benefit in attempting rhythm control in patients at high risk for recurrence of AF. A limitation of this study was that two-thirds of the patients had AF for more than 6 months at the time of entry into the study, and only 23% of the patients in the rhythm control arm were in sinus rhythm after 36 months of follow-up.
HOT CAFÉ
The HOT CAFÉ study was a randomized, multicenter, prospective trial in 205 patients with a clinically overt, persistent first episode of AF.19 The primary endpoint was a composite of death from any cause, thromboembolic complications (especially disabling ischemic stroke), and intracranial or other major hemorrhage. Secondary endpoints included rate control, sinus rhythm maintenance, and discontinuation of therapy, especially if due to a potential proarrhythmic effect, hemorrhage, hospitalization, new or worsening congestive heart failure, or change in exercise tolerance. Patients were followed for a mean of 1.7 ± 0.4 years (maximum follow-up, 2.5 years). Antiarrhythmic drugs used included disopyramide, propafenone, sotalol, and amiodarone. At the end of the study, 63.5% of patients in the rhythm control arm remained in sinus rhythm. Although it was not reported, it is likely that very few, if any, patients in the rate control arm were in sinus rhythm because patients had to be in AF for at least 7 days at entry into the study. There were no sig nificant differences in the primary endpoint between rate and rhythm control patients (odds ratio (OR) 1.98; 95% CI 0.28 –22.3; P > 0.71), nor were there significant differences in secondary endpoints, except that the incidence of hospital admissions was much lower in the rate control than the rhythm control arm (12% vs 74%, respectively; P < 0.001).
Meta-analysis of these studies has demonstrated no significant excess or reduced mortality with either strategy but hinted at a trend towards better survival when rate control was the primary treatment approach (OR 0.87; 95% CI 0.74–1.02; P = 0.09).21
Following these publications, there has been a general movement away from rhythm control in patients who are able to tolerate the arrhythmia when the ventricular rate is adequately controlled. The major limitation of the studies which compared two strategies was inability to achieve a clear difference with respect to rhythm and rate status in the two arms as a significant proportion of patients in the rhythm control arm failed to maintain sinus rhythm and many patients in the rate control arm were in sinus rhythm at the end of the study. A subsequent, retrospective, on-treatment analysis of time-dependent covariates on outcome in the AFFIRM trial has shown that the presence of sinus rhythm conferred a considerable reduction of 47% in the risk of death irrespective of the treatment strategy.22 However, antiarrhythmic drug use was associated with a 42% increase in the risk of death. This has been interpreted as showing that when sinus rhythm is included as a separate factor, the sinus rhythm variable expresses the beneficial effect and the antiarrhythmic drug variable expresses the detrimental effects. Thus, use of antiarrhythmic drugs is associated with increased mortality. However, when sinus rhythm was removed as a separate factor from the analysis, antiarrhythmic drugs were not associated with mortality, presumably because the beneficial antiarrhythmic effects of antiarrhythmic drugs offset their adverse effects. In short, it suggested that in patients with AF, if one could achieve sinus rhythm safely and effectively, sinus rhythm would confer a favorable outcome.
AF CHF
Retrospective studies in patients with left ventricular dysfunction and AF previously showed that there is as high as a twofold increased mortality compared with patients in sinus rhythm.23,24 These and similar observations triggered a large randomized, open-label trial, AF CHF. The AF CHF investigators compared rate and rhythm control strategies specifically in 1376 patients with an ejection fraction of 35% or less and NYHA II–IV heart failure who were followed for a mean of 37 months.15 For patients randomized to the rhythm control arm, aggressive therapy to prevent AF and maintain sinus rhythm was recommended. Amiodarone was the drug of choice for AF suppression and sinus rhythm maintenance, but sotalol and dofetilide were used in selected cases. For patients randomized to the rate control arm, beta-blockers with digitalis were used to achieve the target heart rate of < 80 beats per minute at rest and < 110 beats per minute during a 6-minute walk test. Atrioventricular node ablation and pacemaker therapy were recommended for patients who were otherwise unable to meet rate control targets.
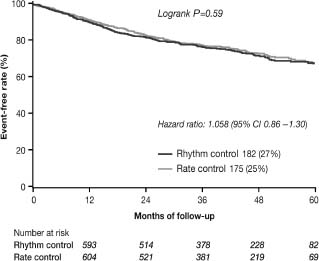
The study showed no benefit of rhythm control on top of optimal medical therapy with regard to the primary endpoint (cardiovascular mortality) as well as prespecified secondary endpoints including total mortality, worsening heart failure, stroke, and hospitalization. Cardiovascular death occurred in 182 (26.7%) of the patients in the rhythm control group compared with 175 (25.2%) in the rate control arm (hazard ratio (HR) 1.058, 95% CI 0.86–1.30; P = 0.59) (Fig. 35.3).15 During the course of the study, 21% of patients crossed over from rhythm to rate control, primarily because of the inability to maintain sinus rhythm, but 10% crossed from the rate control arm to rhythm control, primarily because of worsening heart failure. Similar to the AFFIRM trial, the AF CHF trial conducted a retrospective on-treatment analysis of outcomes and showed that AF does not predict cardiovascular or all-cause mortality once the severity of clinical symptoms and mitral regurgitation is known.24a These results further indicate that rate control is a legitimate primary treatment option for patients with heart failure.
Figure 35.3 Kaplan–Meier estimates of death from cardiovascular causes (primary outcome) in the AF CHF study. AF CHF, Atrial Fibrillation in Congestive Heart Failure. (From Roy et al16, with permission.)
The results of the AF CHF trial likely reflect the considerable improvement in the management of heart failure as well as the management of stroke prevention in patients with AF. In the years since the publication of the retrospective studies which identified a twofold increase in mortality in patients with AF and heart failure compared to patients with sinus rhythm and heart failure, therapy with beta-blockers, afterload reducers, statins, and aldosterone antagonists has considerably improved outcome. We learn once again from the AF CHF trial that because of new, improved and better applied therapies which produce better outcomes, historic controls are often an invalid comparator. A contemporary comparator will reflect the changes, usually the improvements, in patient care that occur over time, which, in turn, will change (usually improve) patient outcomes.
Importantly, the results of rate versus rhythm control studies highlighted the limitations of current therapies to achieve and maintain sinus rhythm. Long-term maintenance of sinus rhythm has proven difficult to achieve in patients with persistent AF, and the method is time-consuming and expensive due to the costs of the antiarrhythmic drugs and the increased need for hospitalization. Thus, the unmet need for safer and more effective antiarrhythmic agents to reverse this trend is clear.
Pharmacologic cardioversion for atrial fibrillation
Pharmacologic cardioversion is considered to be most effective if initiated within 7 days after the onset of the arrhythmia in which case restoration of sinus rhythm can be achieved in nearly 70% of patients, but the success rate decreases significantly as AF persists beyond this limit. The likelihood of spontaneous conversion varies greatly, but is mainly determined by the nature of the arrhythmia (paroxysmal or persistent), the duration of the index episode, the overall duration of AF, the number of recurrences, and the severity of underlying heart disease.25 Systematic analysis of placebo-controlled studies of pharmacologic cardio-version for AF has shown that among patients with AF of less than 24 hours, 66% spontaneously converted to sinus rhythm compared with only 17% of those with the arrhythmia of longer duration (OR 1.8).26
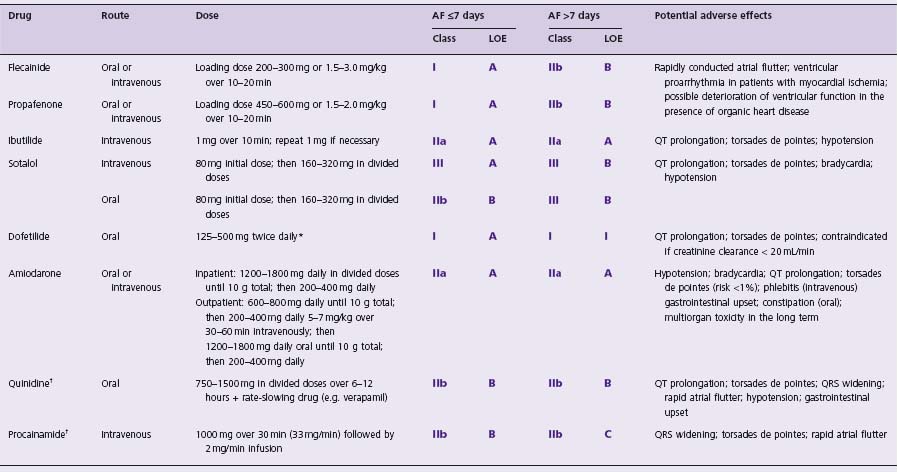
The choice of an antiarrhythmic drug for cardioversion of AF depends on the underlying heart disease.5 Class IC antiarrhythmic agents (propafenone and flecainide), sotalol, and ibutilide are recommended for cardioversion of AF in patients with moderate structural heart disease or hypertension without left ventricular hypertrophy and are contraindicated in patients with a history of heart failure, myocardial infarction with left ventricular dysfunction, and significant left ventricular hypertrophy (>1.4cm). Amiodarone and dofetilide can be used in patients presenting with symptoms of heart failure and known advanced heart disease. Propafenone, flecainide and sotalol are less effective for cardioversion with increased duration of the arrhythmia, and in such patients the choice of drugs is limited to dofetilide, ibutilide and amiodarone. Intravenous formulations of antiarrhythmic drugs are typically used for cardioversion, particularly when the AF is of short duration; however, oral forms of propafenone, flecainide, sotalol and amiodarone are available and may be used in loading doses. Although oral quinidine and oral or intravenous pro-cainamide (class IA antiarrhythmic agents) are available in some countries, there has been a significant decrease in their use worldwide. Antiarrhythmic drugs recommended by the ACC/AHA/ESC guidelines are listed in Table 35.2.
Table 35.2 Antiarrhythmic drugs for pharmacologic cardioversion of atrial fibrillation
AF, atrial fibrillation; LOE, level of evidence;
*dose depends on creatinine clearance: >60mL/min-500mg; 40-60mL/min-250mg; 20-40mL/min-125 mg twice daily; contraindicated if creatinine clearance <20mL/min;
† limited use or withdrawn agents.
Propafenone and flecainide
In placebo-controlled, randomized studies, intravenous propafenone and flecainide were effective in cardioversion of recent (usually 1–72h)-onset AF, with conversion rates as high as 80–90% within an hour after the start of infusion.27–29 The advantage of propafenone and flecainide is the possibility of oral administration for cardioversion of AF; the conversion rates are comparable to those achieved with intravenous formulations, although the effect is usually delayed.30–36 In a meta-analysis of 1843 patients from 27 studies, intravenous or oral propafenone demonstrated a placebo-subtracted efficacy of 31.5% at 4 hours and 32.9% at 8 hours.30 Thus, after administration of a loading single oral dose of propafenone (usually 450–600mg) or flecainide (usually 200–300mg), sinus rhythm was restored in, respectively, 51–59% at 3 hours and 72–78% at 8 hours, compared with respective conversion rates of 18% and 39% on placebo.31 Across the studies, the success rates ranged from 56% to 83% for propafenone and from 57% to 68% for flecainide. The conversion times ranged from 110 ± 59 to 287 ± 352 minutes and 110 ± 82 to 190 ± 147 minutes for propafenone and flecainide, respectively.32–34 Both drugs have been given a Class I recommendation for cardioversion of recent-onset (less than 7 days) AF in patients without significant structural heart disease (Level A). The drugs are considered relatively ineffective for termination of AF of longer duration (Class IIb, Level B).
“Pill in the pocket” approach
The high efficacies of oral propafenone and flecainide in cardioversion of AF have formed the basis for the use of these drugs for termination of the arrhythmia outside the hospital setting. In patients with no or minimal structural heart disease and relatively infrequent (perhaps less than 12 per year) paroxysms of AF of distinct onset which, although symptomatic, do not cause significant hemodynamic compromise (e.g. hypotension), a loading single dose of either drug has proven safe and effective for expedient cardioversion to sinus rhythm.37 In a key feasibility study, a selected cohort of 210 patients who had been successfully treated in hospital with either oral flecainide or propafenone for paroxysmal AF were given a supply of the relevant drug and asked to take a single oral dose within 5 minutes of noticing palpitations. In comparison with their own historic control data, as a result of the “pill in the pocket” strategy, the number of visits to emergency departments fell to 4.9 a month (from 45.6 visits a month during the previous period, P < 0.001), despite the same frequency of arrhythmia episodes.38
However, the experience with this strategy is limited, neither propafenone nor flecainide is licensed for patients to use for self-treating single attacks, and it is mandatory that the efficacy and safety of this strategy are first tested in hospital. It is unknown how valuable the “pill in the pocket” approach will be in the long term because AF tends to progress to sustained over time. Long-term anticoagula-tion will be needed in high-risk individuals. It is generally recommended that patients should not be taking a prophylactic antiarrhythmic drug. There is always the danger of development of atrial flutter with 1:1 atrioventricular conduction due to slowing of the atrial rate, associated with dangerous widening of the QRS complex (due to the enhanced (use-dependent) sodium channel blocking effect) and rarely left ventricular dysfunction secondary to the negative inotropic effect. Atrial flutter was reported in 2.5–20% of patients who received oral loading doses of propafenone or flecainide for conversion of AF (5–7% on average), and 2–14% of patients developed hypotension.34 Therefore, concomitant administration of an atrioventri-cular node-blocking agent (a beta-blocker, verapamil or diltiazem) is warranted. Transient atrioventricular block, QRS widening, and left bundle branch block occurred in approximately 2–8%. Class IC drugs usually are ineffective for conversion of atrial flutter. They slow conduction within the re-entrant circuit and prolong the atrial flutter cycle length, but rarely interrupt the circuit and there is greater risk of 1:1 atrioventricular conduction. The efficacy rates have been reported to be as low as 13–40%.39
Sotalol
Intravenous sotalol generally is ineffective for acute cardio-version of AF of any duration (Class III, Level A). The conversion rate in one study was 11–13% with sotalol vs 14% on placebo in the double-blind phase and 30% in the open-label phase.40 In a direct comparison study with ibutilide in 308 patients with AF or flutter of 3 hours to 45 days, sotalol 1.5 mg/kg (n = 103) restored sinus rhythm in only 11% of patients with AF and 19% of patients with flutter and was significantly inferior to ibutilide.41 Even among patients with very recent onset AF (<48 hours), only 12% converted to sinus rhythm at 3 hours with oral sotalol 80–160 mg and a further 12% converted to sinus rhythm at 8 hours after receiving a cumulative dose of 160–240 mg.42 In this study, the conversion rates for sotalol were significantly lower than for oral quinidine in combination with digoxin (36% and 71% at 3 and 8 hours, respectively). In the Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T), 24.2% of patients with persistent AF treated with sotalol converted to sinus rhythm within 28 days, compared with 27.1% on amiodarone and only 0.8% on placebo.43 Sotalol may offer a modest benefit of facilitating spontaneous conversion to sinus rhythm and, in addition, can ensure ventricular rate control in patients awaiting electrical cardioversion (Class IIb, Level B).
The antifibrillatory effect of sotalol is limited by reverse use dependency of its effect on atrial refractoriness: sotalol prolongs the atrial effective refractory period at normal and slow atrial rates, but not during rapid AF. The adverse effects of sotalol include hypotension, bradycardia, QT interval prolongation and associated ventricular proar-rhythmia (torsades de pointes). Bradycardia and hypotension were the most common with intravenous sotalol, with an incidence of 6.5% and 3.7%, respectively.43 Polymorphic ventricular tachycardia or torsades de pointes was reported in 1% of men and 4.1% of women who received sotalol for ventricular tachyarrhythmias.44 The risk of proarrhythmia is increased in the presence of left ventricular hypertrophy because of increased duration and inhomogeneity of repo-larization in the hypertrophied myocardium, increased transmural dispersion of repolarization, disruption of gap junction coupling, and a greater likelihood of ischemia and fibrosis. Sotalol levels are also increased in renal failure and torsades de pointes is more likely under these conditions.
Ibutilide
In randomized, placebo-controlled studies and direct comparisons with procainamide and sotalol, the class III drug ibutilide has proven more effective for cardioversion of atrial flutter than AF.41,45–47 The overall conversion rates for atrial flutter were reported to be almost twofold higher than for AF (56–70% vs 31–44%).41,46 In direct comparison studies, ibutilide 2 mg cardioverted 70% of atrial flutter compared with only 19% on sotalol41 and 14% on procain-amide.47 Higher doses of ibutilide administered as a single bolus of 2 mg or two successive infusions of 1 mg are usually required for termination of AF. Thus, in the Ibuti-lide/Sotalol Comparator Study, patients with AF were twice as likely to convert to sinus rhythm after receiving a 2 mg infusion than those who received a 1 mg infusion (44% vs 20%).41 The antiarrhythmic effect of ibutilide decreased if the arrhythmia had persisted for more than 7 days–from 71% to 57% for atrial flutter and from 46% to 18% for AF. Nevertheless, ibutilide is recommended for cardioversion of AF (Class IIa, Level A).
Unlike sotalol and procainamide (a class IA agent), ibu-tilide does not produce significant hypotension but like many of the class III antiarrhythmic drugs, it may cause QT interval prolongation and ventricular proarrhythmia. In the ibutilide trials, the incidence of polymorphic ventricular tachycardia or torsades de pointes requiring electrical cardioversion was 0.5–1.7% and the incidence of self-terminating polymorphic ventricular tachycardia was 2.6–6.7%.48 There are insufficient data to support the use of ibutilide in patients with significant structural heart disease as many controlled studies of ibutilide have only enrolled patients with mild or moderate underlying heart disease. Ibutilide 2 mg administered intravenously on top of long-term therapy with oral amiodarone in patients referred for electrical cardioversion terminated AF in 39% of patients and flutter in 54%.49 The QT interval was significantly prolonged, but there was only one case of non-sustained polymorphic tachycardia.
Dofetilide
In small (less than 100 patients) randomized, double-blind, placebo-controlled studies, dofetilide administered intravenously at 8μ g/kg restored sinus rhythm in about one-third of patients presenting with atrial flutter or AF compared with 0–3.3% on placebo.50,51 As with ibutilide, the conversion rates were significantly higher for atrial flutter than for AF (54–64% vs 14.5–24%).50,51 In a double-blind, dose-ranging, pharmacokinetic study, dofetilide 8μg/kg was confirmed as the most effective dose with an acceptable rate of adverse events: 39% of patients in the dofetilide group converted to sinus rhythm compared with 6% in the placebo group.52 In line with the conversion rates observed with other antiarrhythmic agents, the likelihood of pharmacologic conversion on dofetilide decreased as the duration of AF increased: the drug was effective in 67% of patients with arrhythmia onset within 24 hours of treatment, 36% of patients with AF of 1–7 days, and 24% of patients with AF of more than 7 days.
Two medium-size prospective studies, SAFIRE-D (Symptomatic Atrial Fibrillation Investigative Research on Dofetilide) and EMERALD (European and Australian Multicenter Evaluative Research on Dofetilide), reported a modest 30% cardioversion rate of persistent AF with high-dose (1000μ g daily) oral dofetilide compared with 1.2% of spontaneous conversion on placebo53 and 5% on sotalol.54 In the pooled analysis from the DIAMOND (Danish Investigations of Arrhythmia and Mortality ON Dofetilide) studies in patients with symptomatic heart failure and an ejection fraction ≤35% (DIAMOND-CHF) or myocardial infarction with left ventricular dysfunction (DIAMOND-MI), oral dofetilide had a neutral effect on mortality and also demonstrated a greater rate of conversion to sinus rhythm (44% vs 14%).24
Oral dofetilide is considered safe and relatively effective for pharmacologic cardioversion of AF including arrhythmia duration of more than 7 days (Class I, Level A). Intravenous dofetilide is rarely used for pharmacologic cardioversion. However, dofetilide has been reported to prolong the QT interval and cause torsades de pointes. This effect is dose related. Therefore, it is mandatory that dofetilide be initiated in hospital and that patients should be monitored for 3 days. In addition, the dose of dofetilide requires adjustment to creatinine clearance.
Amiodarone
Amiodarone is currently assigned a Class IIa, Level A indication for cardioversion of AF of any duration. Meta-analysis of 13 randomized controlled studies in 1174 patients has shown that IV amiodarone was 44% more effective in converting AF compared with placebo, but its antiarrhythmic effect was delayed by 24 hours.55 At 8 hours, the probability of restoration of sinus rhythm was 65% higher with flecainide or propafenone than with amiodarone. In a double-blind direct comparison with dofetilide, amiodarone cardioverted only 4% of patients with AF compared with 35% on dofetilide and 4% on placebo.56 In one study, amiodarone administered intravenously as a bolus of 300 mg for 1 hour followed by infusion of 20 mg/ kg for 24 hours and oral treatment for 4 weeks cardioverted 80% of AF.57 However, spontaneous conversion rates in this study were also high (40%). Amiodarone given at a high single loading oral dose of 30 mg/kg (approx. 2.4 g) was less effective than oral propafenone 600 mg at 4 hours (16% vs 37%) and comparable with propafenone at 24 hours (56% vs 47%), but the median time to conversion to sinus rhythm was significantly shorter with propafenone than amiodarone (2.4 vs 6.9 hours).58
Intravenous amiodarone has a modest effect on atrial refractoriness and therefore is moderately effective in terminating the arrhythmia in the emergency setting, but unlike class IC agents, amiodarone does not have any negative inotropic effect, controls the ventricular rate, and is associated with a low incidence of torsades de pointes. All this makes it safe to use in patients with advanced structural heart disease. Amiodarone prolongs the QT interval but, unlike pure class III agents, exhibits a low arrhythmogenic potential (less than 1%).59 The most common effects of intravenous amiodarone are hypotension and relative bradycardia. There is limited experience with the use of intravenous amiodarone for termination of AF in critically ill patients.60
The mortality and morbidity study CHF-STAT (Congestive Heart Failure Survival Trial of Antiarrhythmic Therapy) in 667 patients with a mean ejection fraction of 25% has shown that long-term treatment with oral amiodarone 400 mg daily for the first year and 300 mg daily for the remainder of the 4.5-year trial had a neutral effect on survival but was associated with greater rates of conversion to sinus rhythm compared with placebo (31% vs 7.7%).61 The SAFE-T study randomized 665 patients with persistent AF (74–80% less than 1 year) to therapy with amiodarone, sotalol or placebo for 28 days prior to carrying out electrical cardioversion.43 The amiodarone regimen for this period was 800 mg for the first 2 weeks and 600 mg for the next 2 weeks. Of 267 patients who received amiodarone, 27.1% converted to sinus rhythm compared with 0.8% out of 137 in the placebo group. In a study of 95 patients with a mean AF of almost 2 years, amiodarone prescribed 600 mg daily for 4 weeks restored sinus rhythm in 34% of patients compared with 0% in the placebo group.62
Procainamide, quinidine, and disopyramide
The class IA drug procainamide has been shown to facilitate conversion of AF of less than 48 hours, but its efficacy is limited in AF of longer duration.47,63–65 The conversion rates with procainamide administered intravenously at a dose 1000–1200 mg over 30 minutes ranged from 21% to 70%. In direct comparison studies, the efficacy of procainamide was comparable to that of propafenone (69.5% vs 48.7%)64 and flecainide (65% vs 92%).65 In a randomized, single-blind, placebo-controlled study in 362 patients with AF of less than 48 hours, the conversion rates with IV procainamide, propafenone, and amiodarone were 68.5%, 80.2%, and 89.1%, respectively.66 Propafenone produced the most rapid effect, with a median time to conversion of 1 h, followed by procainamide (3 h) and amiodarone (9 h). The non-target effects of procainamide are vasodilation and hypotension due to the alpha-adrenergic properties, anticholinergic action, atrioventricular node blockade, worsening heart failure, and lupus erythematosus. The drug is no longer used routinely for cardioversion of AF (Class IIb, Level B).
Quinidine given orally in a cumulative dose of up to 1200–2400 mg over 24 hours has been shown to cardiovert 60–80% of recent-onset AF; it is more effective than sotalol67 and in some studies it was as effective as intravenous amiodarone.68 The effect usually occurs within 12 hours of treatment. Quinidine poses increased risk of torsades de pointes which is observed at a rate of 6%; it widens the QRS complex, causes gastrointestinal side effects in a significant proportion of patients, and is contraindicated in individuals with structural heart disease. The poor safety profile of quinidine has led to a decrease in its use for acute cardio-version of AF (Class IIb, Level B).
There is limited evidence for the efficacy of intravenous disopyramide for acute pharmacologic cardioversion in patients with AF. In one study, disopyramide administered as a bolus of 2 mg/kg over 5 minutes restored sinus rhythm in 10 of 14 (71%) patients with self-limiting lone AF and 3 of 7 (43%) patients with atrial flutter.69 There are concerns, however, that the adverse effects such as proarrhythmia, hypotension, asystole, and non-target effects resulting from anticholinergic activity of disopyramide may offset its modest antiarrhythmic potential (Class IIb, Level B).5
Other agents
One-quarter to one-half of patients with AF have low serum magnesium levels.70 The electrophysiologic effects of magnesium include prolongation of the atrial and atrio-ventricular node refractory periods which are essential determinants of ventricular rates and maintenance of AF. Meta-analysis of eight studies in 476 patients showed that magnesium sulfate, administered intravenously at an initial dose of 1200–5000mg over 1–30 minutes (in some studies followed by a second dose or continuous infusion for 2–6 hours), was superior to placebo or the active comparator in cardioverting AF with an odds ratio of 1.6 (95% CI 1.07–2.39).70 Time to conversion was 3.8–14.9 hours and the most common side effects were sensations of warmth and flushing. In addition to its antifibrillatory action, magnesium produced effective ventricular rate control. There was no correlation between serum magnesium levels and the response. However, magnesium is not routinely used for pharmacologic cardioversion of AF (Class IIb, Level B), but it may potentiate the effect of other antiarrhythmic agents, e.g. in patients with refractory AF.
Digitalis, beta-blockers, and calcium antagonists usually are ineffective for acute conversion of AF (Class III).27,71,72 The DAAF (Digitalis in Acute Atrial Fibrillation) study has shown no difference in cardioversion rates at 16 hours between intravenous digoxin and placebo (51% vs 46%).73 Moreover, the drug has been shown to have a profibrillatory effect due to its cholinergic effects which may cause a non-uniform reduction in conduction velocity and effective refractory periods of the atria, and to delay the reversal of remodeling after restoration of sinus rhythm.74 Digoxin is not indicated for pharmacologic cardioversion of AF (Class III, Level A). Short-acting intravenous beta-blockers (e.g. esmolol) and non-dihydropyridine calcium antagonists (verapamil and diltiazem) are more commonly used for rate control than for restoration of sinus rhythm.
Antiarrhythmic drugs and electrical cardioversion
Antiarrhythmic drugs can be used to facilitate electrical cardioversion and to prevent immediate or early recurrence of AF (Class IIa, Level B). Synergistic action of anti-arrhythmic drugs may be due to prolongation of atrial refractoriness, conversion of AF to a more organized atrial rhythm (e.g. flutter) which may be cardioverted with less energy, the suppression of atrial premature beats that may re-initiate AF, and prevention of atrial remodeling. The disadvantages are increased risk of ventricular proarrhyth-mia and bradycardia.
Pretreatment with ibutilide lowered the energy requirement by approximately 30% and improved the success rate of cardioversion, including those who previously had failed electrical cardioversion.75 Flecainide administered intravenously increased the likelihood of converting to sinus rhythm by 16% and significantly reduced the energy requirement.76 Intravenous sotalol was synergistic with internal electrical cardioversion by preventing reinitiation of AF. Sotalol suppressed atrial premature beats and prolonged the coupling interval of atrial premature beats, thus reducing risk of early recurrence.77 Higher success rates of electrical cardioversion for persistent AF were reported in patients pretreated with oral amiodarone compared with diltiazem (88% vs 56%).78 The results with calcium antagonists were controversial. Thus, in the VEPARAF (VErapamil Plus Antiarrhythmic drugs Reduce Atrial Fibrillation) trial, adjunctive treatment with verapamil reduced the AF recurrence rate from 35% to 20%.79 In the VERDICT (Verapamil versus Digoxin Cardioversion Trial), only 47% of patients in the verapamil-treated group and 53% of those in the digoxin-treated group remained free from recurrence of the arrhythmia during the first month after cardioversion.80
New agents for pharmacologic cardioversion
Vernakalant
Vernakalant is a new arrhythmic drug agent with an affinity for ion channels specifically involved in the repolarization processes in atrial tissue, in particular, the ultrarapid potassium repolarization current IKur. Although IKur current is the main target of vernakalant, its mechanism of action involves blockade of several ion channels such as Ito, and INa, but there is little impact on major currents responsible for ventricular repolarization, such as IKr and IKs currents. The intravenous formulation of vernakalant is under regulatory body review for pharmacologic cardioversion of AF.
The efficacy of vernakalant was investigated in one dose-finding study, three medium-size randomized clinical studies, and a phase IV open-label study (Table 35.3).81–84 In the randomized, double-blind, placebo-controlled Atrial arrhythmia Conversion Trials (ACT I and II), vernakalant was significantly more effective than placebo in converting AF of less than 7 days (51.7% and 51.2% compared with 4% and 3.6%, respectively, P < 0.001).82,83 Vernakalant was administered as a 10-minute infusion of 3 mg/kg and if AF persisted after 15 minutes, a second infusion of 2 mg/kg was given. The median time to conversion was 8–11 minutes and the majority of patients (75–82%) converted after the first dose. The highest efficacy was observed for AF of up to 72 hours (70–80%). The results were reproduced in the open-label ACT IV study in which vernaka-lant restored sinus rhythm in 50.9% within 14 minutes after the start of treatment. Vernakalant was significantly less effective in converting AF of more than 7 days and did not convert atrial flutter.
Table 35.3 Summary of clinical studies of vernakalant in atrial fibrillation
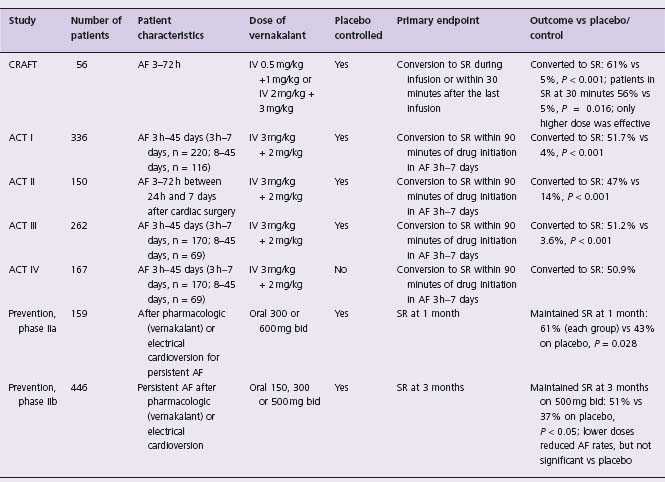
ACT, Atrial arrhythmia Conversion Trial; A F, atrial fibrillation; CRAFT, Controlled Randomized Atrial Fibrillation Trial; I V, intravenous; SR, sinus rhythm. Reproduced with permission from Savelieva et al.84
The drug was well tolerated, with no significant QT prolongation or drug-related torsades de pointes. However, in the ACT I study, the QTc values after infusion were greater in the vernakalant group than in the placebo group, and 24% of patients in the vernakalant group had QTc >500ms as opposed to 15% in the placebo group, but no torsades de pointes was reported during the first 24 hours after infusion.82 The most common (> 5%) side effects of vernaka-lant were dysgeusia, sneezing, and nausea.
Prevention of atrial fibrillation
Prophylactic antiarrhythmic drug therapy generally is not recommended after a first episode of AF, either self-terminating or after pharmacologic or electrical cardioversion.5 However, this approach can be appropriate in a small proportion of patients as paroxysmal AF tends to become persistent or permanent. After cardioversion, approximately 25–50% patients will have recurrence of AF within the first 1–2 months (early recurrence).85 Thereafter, the recurrence rate is about 10% per year (late recurrence). Prophylactic antiarrhythmic drug therapy is recommended for the vast majority of patients with paroxysmal AF when paroxysms occur frequently and are associated with significant symptoms or lead to worsening left ventricular function, and for patients with persistent AF when the likelihood of maintenance of sinus rhythm is uncertain, especially in the presence of risk factors for recurrence such as left atrial enlargement, evidence for depressed atrial function, left ventricular dysfunction, underlying cardiovascular pathology, long duration of the arrhythmia, advanced age, and female gender) (Class I, Level A).
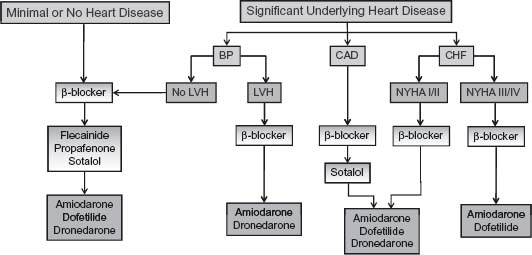
The choice of antiarrhythmic drug for prevention of AF is dictated by the presence and nature of underlying heart disease (Fig. 35.4).
A systematic review of 44 studies in 11 322 patients has shown that antiarrhythmic drugs significantly reduced AF recurrence after cardioversion; the number of patients needed to treat to prevent a recurrence ranged from two to nine depending on the agent used.86 Antiarrhythmic drugs may also render AF less symptomatic, less frequent, and less sustained or may promote conversion to sinus rhythm. The majority of large, high-quality studies were conducted in patients with persistent AF, i.e. AF requiring electrical or pharmacologic therapy to terminate. This was probably because the recurrence of a persistent episode is more “predictable,” is likely to occur during the first year, and is easier to recognize and document, especially when the time to first symptomatic recurrence is used as an end-point. Hence, the results of many trials of the efficacy of antiarrhythmic drugs for the prevention of recurrences of persistent AF were extrapolated on the patient populations with a paroxysmal (self-terminating) arrhythmia.
Beta-blockers
Beta-blockers generally are modestly effective in preventing AF (Class IIb, Level B) and are mainly used for rate control. An exception is AF caused by thyrotoxicosis or, rarely, lone adrenergically mediated AF when beta-blockers may be a first-line therapy (Class I).87,88 In anecdotal reports beta-blockers were superior to placebo or were as effective as sotalol for prevention of persistent AF.89,90 In a medium-size (n = 394), double-blind, placebo-controlled study, treatment with metoprolol 100 mg daily was associated with a modest reduction in arrhythmia recurrence at 3 months after electrical cardioversion from 60% in the placebo arm to 48% in the metoprolol arm.89 Patients who received metoprolol had slower ventricular rates during the recurrence of AF and were probably less symptomatic. In another study, 58% of patients treated with bisoprolol 5 mg daily and 59% of patients treated with sotalol 160 mg daily maintained sinus rhythm at 1 year after cardioversion.90
Some beta-blockers such as carvedilol may be more potent antiarrhythmics because of their direct effects on cardiac ion channels beyond their antiadrenergic action. In addition, carvedilol has antioxidant activity and may protect the atrial myocardium from oxidative injury caused by fast atrial rates and ischemia. In direct comparisons, carvedilol demonstrated no benefit over bisoprolol, although a higher proportion of patients treated with carvedilol completed the 1-year study in sinus rhythm (68% v 54%)91 although this trend was not significant and the number of patients was small (n = 90). Beta-blockers may, however, prevent AF associated with congestive heart failure: a meta-analysis of seven studies in 11 952 patients has shown that therapy with beta-blockers, on background therapy with ACE inhibitors and diuretics, was associated with a statistically significant reduction in the incidence of new-onset AF from 39 to 28 per 1000 patient-years (a 27% reduction in relative risk) during mean follow-up of 1.35 years (Class I, Level A).92
Quinidine
Quinidine has been used for treatment of AF since the discovery of its antiarrhythmic properties in early 1920s. In a meta-analysis of six randomized controlled trials in 808 patients, published in 1990, the proportions of patients remaining in sinus rhythm at 3, 6, and 12 months were 69%, 58%, and 50% in the quinidine group compared with 45%, 33%, and 25% in the control group.93 In a 2006 analysis which included two recent large-scale studies of quinidine, PAFAC (Prevention of Atrial Fibrillation After Cardio version) and SOPAT (Suppression Of Paroxysmal Atrial Tachyarrhythmias), quinidine reduced the incidence of recurrent AF by 49%.86 The antiarrhythmic effect of quinidine was offset by high all-cause mortality and sudden death in the quinidine-treated patients compared with controls (2.9% vs 0.8%; OR 2.98).93 The foremost safety issue for quinidine is its propensity to cause ventricular proarrhythmia including torsades de pointes, even at low or subtherapeutic doses. In earlier studies, the incidence of quinidine-induced ventricular pro-arrhythmias was reported to be 15%.12
Following the reports of increased mortality associated with quinidine and the development of newer antiarrhythmic drugs which are better tolerated, the use of quinidine has significantly decreased worldwide. However, the PAFAC94 and SOPAT95 trials showed no adverse effect on mortality, probably because in these studies, quinidine was used at lower doses (320–480 mg as opposed to 1000–1800 g daily in the previous trials93), in combination with vera-pamil, and in patients with less significant structural heart disease. The number of patients with previous myocardial infarction (2.9–5%) and coronary artery disease (17–21%) was low.
In the PAFAC (848 patients with persistent AF) and SOPAT studies (1012 patients with similar clinical characteristics), which both employed daily transtelephonic ECG monitoring for arrhythmia detection, quinidine was superior to placebo and was as effective as sotalol in maintenance of sinus rhythm after cardioversion. The overall efficacy of antiarrhythmic drug therapy was modest: at 1 year, only about 30–35% of patients had no AF recurrence (including asymptomatic episodes detected during daily transtelephonic ECG transmissions) compared with 20–23% in the placebo arm. As for the safety concerns, there were five reports of ventricular tachycardia of more than 10 beats in patients treated with quinidine, four in the PAFAC study and one in the SOPAT study, but no torsades de pointes, which did occur in the sotalol arm of the PAFAC study. Nevertheless, the modest efficacy of quinidine and concerns about its safety have led to the withdrawal of quinidine from the list of drugs available for treatment of AF (Class III, Level A).
Disopyramide
Disopyramide is rarely used for treatment of AF because of its negative inotropic effect, the torsadogenic potential, and poor tolerance due to antimuscarinic properties. However, the use of disopyramide is advocated in patients with lone, vagally mediated AF (Class IIa, Level B). Although it was commonly prescribed for ventricular arrhythmias, data on the efficacy of disopyramide in AF are sparse. In a randomized, double-blind placebo-controlled study of 90 patients, disopyramide maintained sinus rhythm in 70% of patients at 1 month and 54% of patients at 1 year after cardioversion; the corresponding values for placebo were 39% and 30%, respectively.96 In a small, double-blind study of 56 patients, disopyramide 250 mg tid was not significantly different from propafe-none 300 mg tid in maintaining sinus rhythm at 6 months after cardioversion (67% vs 55%) but was associated with a significantly higher withdrawal rate due to adverse events, including two cases of heart failure and fast ventricular rates during AF in one patient.97
Propafenone and flecainide
Propafenone and flecainide are recommended as first-line therapy (Class I, Level A) for AF in patients without significant structural heart disease, i.e. patients without congestive heart failure, left ventricular dysfunction, hypertrophy, previous myocardial infarction or coronary artery disease. Both propafenone and flecainide reduced the recurrence rate by two-thirds, with no advantage of one drug over the other.86 A number of randomized, controlled studies addressed the long-term efficacy of class IC antiar-rhythmic drugs in AF.98–104 In the Propafenone Atrial Fibrillation Trial (PAFT) of 102 patients, the likelihood of maintenance of sinus rhythm at 6 months after cardioversion was 67% with low-dose propafenone (450 mg) compared with 35% on placebo, with no increase in drug-related side effects (10% on propafenone vs 14% on placebo).99 The UK PSVT (Paroxysmal Supraventricular Tachycardia) study evaluated the safety and efficacy of propafenone, 600 mg and 900 mg daily, and showed that both regimens were effective but a dose of 900 mg was associated with a less favorable adverse events profile.100
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree