Device Specifics
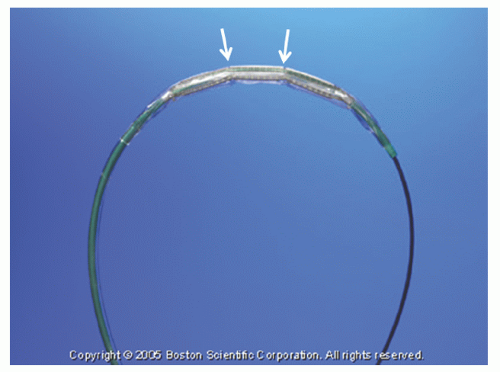
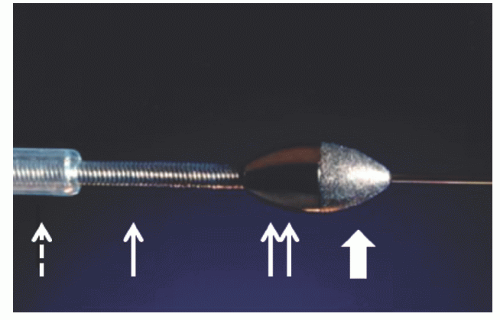
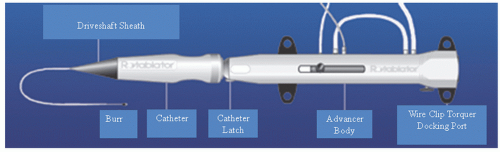
The Rotalink® burr catheter (Boston Scientific, Boston, MA) consists of an elliptical, nickel-coated brass burr attached to a hollow flexible 4.3F drive shaft, which is encased in a Teflon sheath (
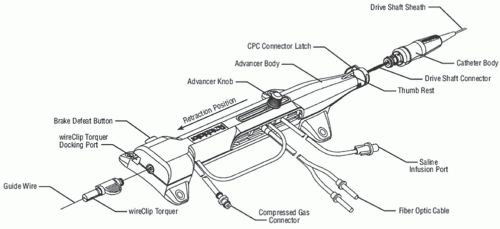
Figure 29.1). The sheath protects the artery proximal to the lesion from the rotating drive shaft and allows flush solution to be pumped to lubricate the drive shaft and burr. The burr’s ablative distal surface is embedded with 20 µm diamond chips, with 5 µm protruding from the surface. The proximal nonablative surface of the burr is smooth. The back end of the Rotalink® burr catheter is connected to a Rotalink® advancer, which allows the operator to extend and retract the burr within the vessel (
Figures 29.2 and
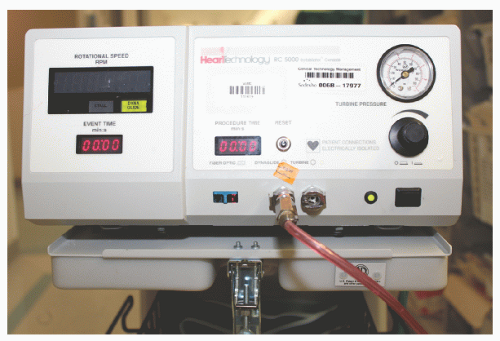
29.3). A control console delivers air or nitrogen through a pneumatic hose to the turbine housed within the Rotalink® advancer to spin the drive shaft and the burr (
Figures 29.4 and
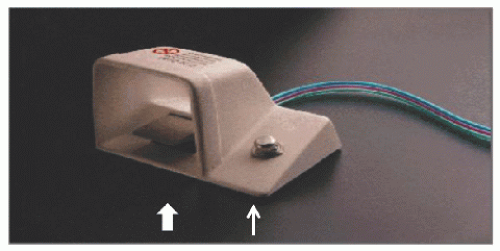
29.5). The console is activated by a foot pedal (
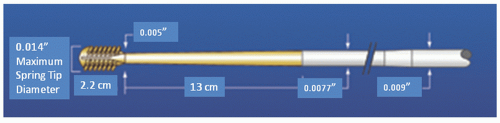
Figure 29.6); turbine pressure is adjusted by a control knob; and rotational speed is monitored by a fiberoptic tachometer. The RotaWire™ guidewires combine a 0.009-inch diameter body with a 0.014-inch tip (
Figure 29.7), and are supplied with a specific wire clip to facilitate wire manipulation (
Figure 29.8). The burr can be advanced over the 0.009-inch section, but its forward movement is delimited by the wider wire tip. During turbine activation, a wire brake is engaged to prevent spinning of the guide wire, which could otherwise traumatize the distal vessel. The wire clip provides a secondary brake. The RotaWire™ guidewires have no lubricious coating, no shaping ribbon, and are easily kinked.
Technique
The selection of PTRA depends on specific characteristics of the lesion and the patient. Generally, this technique is not applied in acute myocardial infarction, thrombotic lesions, coronary dissections, or saphenous vein grafts (SVG) with poor distal runoff, nor in the setting of severe left ventricular dysfunction. Patients are pretreated with aspirin and possibly a calcium channel blocker to counteract PTRA-induced vasospasm. The role of thienopyridines with PTRA has not been rigorously studied, but glycoprotein (GP) IIb/IIIA receptor antagonists have shown benefit in limiting speeddependent platelet activation.
4,
5 Appropriate anticoagulation is instituted. Some operators prefer unfractionated heparin over bivalirudin to facilitate reversal of anticoagulation in the event of vessel perforation, although bivalirudin has been used with similar clinical outcomes.
6 Rotaglide™, a lipid emulsion, can be added to the flush solution to reduce friction, limit heat generation, and facilitate device deliverability.
This should not be used in patients who are allergic to egg products or olive oil. Various combinations of vasodilators are often added as well to counteract vasospasm and microvascular no-reflow. Typical “RotaFlush” solutions mix 4 mg of nitroglycerin and 5 mg of verapamil in 500 mL of saline. A temporary pacing wire is generally utilized in PTRA of the right coronary or dominant circumflex owing to the risk of profound bradycardia, which is believed to result from adenosine release with red cell fragmentation.
A guiding catheter with a gentle curve and an inner diameter at least 0.004 inch longer than the anticipated largest burr diameter is recommended, to minimize resistance to device advancement. Complex lesions are often difficult to cross with rotablator wires owing to their poor torquability. In such cases, a conventional exchange length angioplasty wire is used to cross and then exchanged for the rotablator guidewire using a suitable transport or low-profile balloon catheter. Typically, a RotaWire™ floppy is chosen in order to minimize guidewire bias—a phenomenon observed when a stiff guidewire straightens a curved vessel segment and causes deeper cuts or dissection as the burr is forced against the tautly stretched lesser curvature of the vessel. On the other hand, the floppy guidewire may fail to adequately constrain the burr’s passage around tight bends, leading to uncontrolled cutting on the greater curvature of the vessel. The RotaWire™ extra-support wire is generally utilized for some distal or very heavily calcified lesions. Burrs for coronary use are available in 1.25 to 2.5 mm diameters. The selection of burr size is largely empirical, but the final burr-to-artery ratio should generally not exceed 0.7 (e.g., 2.15-mm burr in a 3.0-mm vessel).
7,
8 In treating long segments of disease, heavily calcified lesions, and subtotal de novo lesions, it is generally advisable to start with a smaller (1.5 or 1.75 mm) burr and step up to the final burr size in 0.5-mm increments.
Once the guidewire is placed across the lesion, the burr should be advanced to within a few centimeters of the rotating hemostatic valve, with the lines for compressed air supply and tachometer readout attached to the drive console and the advancer lever locked in its midway position. The compressed air or nitrogen source to the console is confirmed to have a pressure of at least 500 PSI. A preprocedural “DRAW” checklist, consisting of the following steps, is then applied: (i) “Drip”—Adequate flow of the pressurized heparinized flush through the Teflon sheath is visualized; (ii) “Rotation”—While the operator holds the catheter carefully so that the burr tip is not in contact with the sterile drapes, the system should be tested by depressing the foot pedal and having an assistant adjust the turbine pressure to achieve the desired burr speed; (iii) “Advancer”—Test whether the advancer moves the burr freely; (iv) “Wire”—Ensure that the wire clip is in place on the wire and test whether the brake locks the wire in place during rotation. Once this test has been completed, the static burr can be advanced over the wire into and through the guiding catheter. Any resistance encountered as the burr is passed around the primary curve of the guiding catheter can be overcome by firm traction on the guidewire or gentle traction on the guiding catheter itself to lessen the curve slightly. It may be noted, however, that the guiding catheter must remain well seated in the vessel ostium to prevent kinking or looping of the guidewire in the aortic root while the burr is advanced— such unrecognized loops in the radiolucent wire can lead to its transection when the burr is activated at the ostium.
Once the burr has been advanced to 1 to 2 cm proximal to the target lesion, the advancer lever should be unlocked and pulled gently back to near its proximal limit as the entire catheter is withdrawn gently by 1 or 2 mm. This relieves any
compression in the drive shaft that might otherwise cause the burr to lurch forward into the lesion on activation. Under fluoroscopy, the burr is then activated by the foot pedal and adjusted to the desired “platform” speed (generally 160,000 to 180,000 rpm for burrs ≤2.0 mm, 140,000 to 160,000 rpm for burrs >2.0 mm) before engaging the lesion. Advancement of the lever then brings the spinning burr slowly into contact with the lesion. It is important to be aware of the sound of the turbine, the rotational speed display, and tactile feedback during rotablation. When the burr face encounters excessive resistance to rotation, the speed will fall, but it is essential to avoid speed drops of >5,000 rpm during advancement.
8 Larger speed drops caused by excessive pressure on the burr against the lesion may result in the liberation of larger particles, frictional heating of the plaque, or torsional dissection. We prefer advancing with a “pecking” motion in which brief (1 to 3 seconds) periods of plaque contact are alternated with longer (3 to 5 seconds) periods of reperfusion provided by pulling the burr back from the plaque face. This reduces speed drops and aids in the clearance of particulate debris through the distal circulation. Some operators favor intermittent injections of dilute contrast through the guide during the burr run to monitor for vessel complications and to enhance clearance of particulate debris.
After a brief run (usually <30 seconds of operation), the device should be withdrawn into the proximal vessel and rotation suspended for a similar time before reactivating and advancing the burr again. During each pause, a small test injection should be performed to ensure antegrade flow and absence of vascular trauma or perforation. This sequence should be repeated until the device can be advanced through the full length of the lesion without any fluoroscopic or tactile resistance to burr advancement and with no audible change in the pitch of the turbine or reduction in burr speed. The foot pedal is then used to activate the lower speed “dynaglide” mode, and the burr is removed while depressing the brake-release button. It is important to note that in the dynaglide mode the burr is not subject to pressure control (spinning at 90,000 rpm in all circumstances) and should never be advanced in this mode.
Lesion Selection for Rotational Atherectomy
Based on the failure of atheroablation to improve clinical outcomes, current PCI guidelines do not recommend routine use of PTRA. With the superiority of DES firmly established over other percutaneous revascularization techniques, the primary role of PTRA is to facilitate delivery and expansion
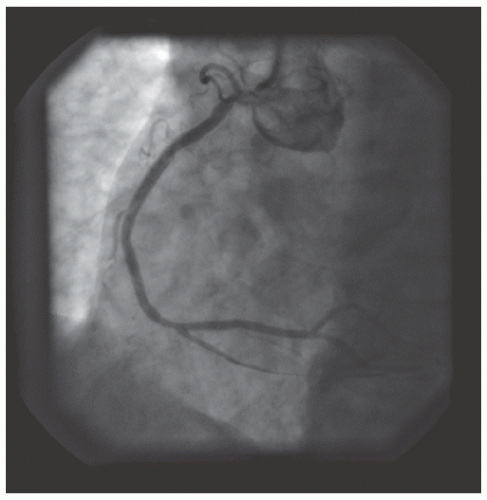
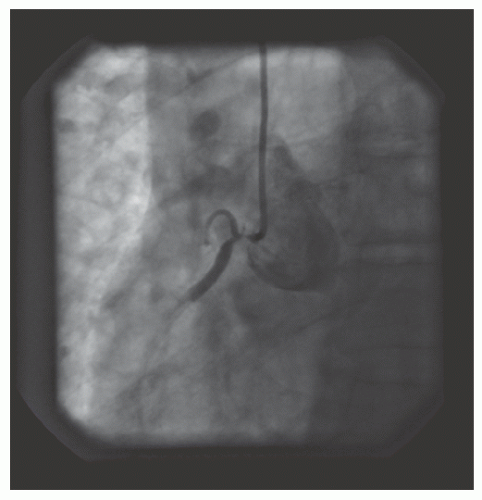
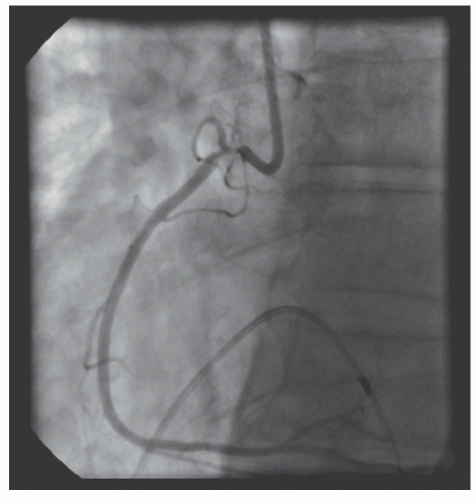
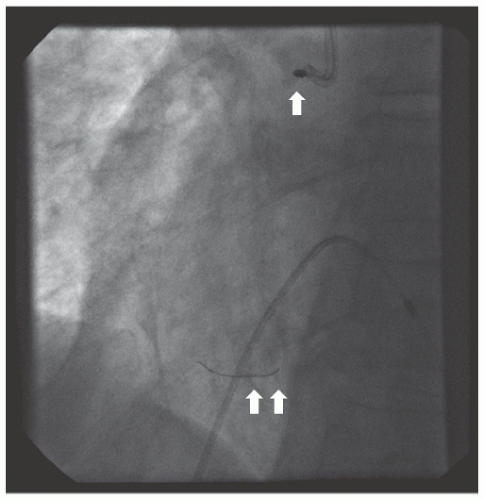
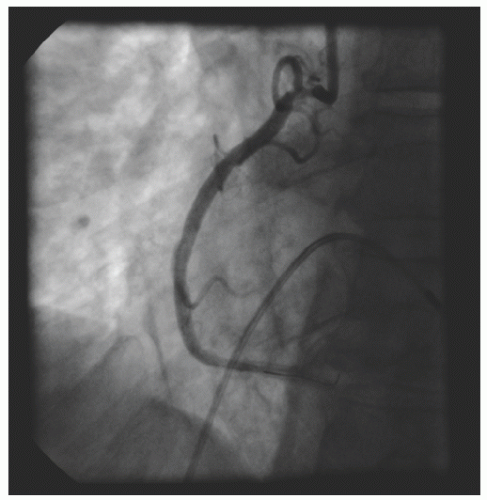
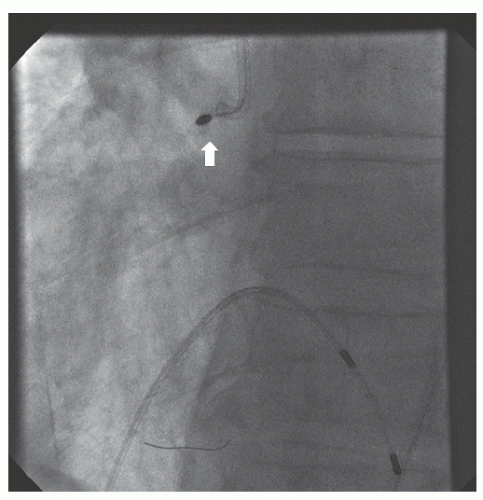
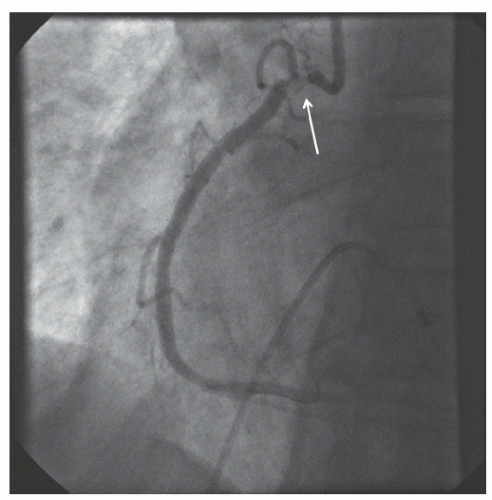
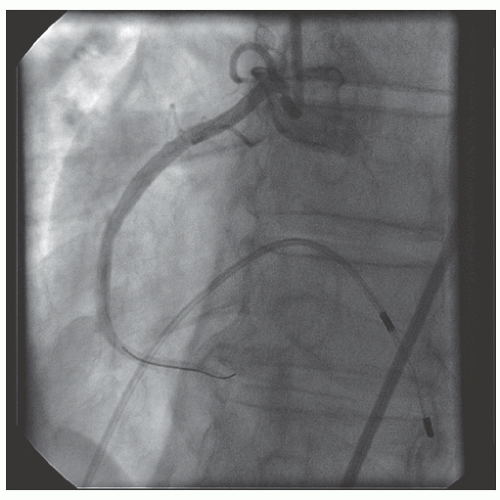
of DES. Heavily calcified lesions are the most common indication for PTRA. An illustrative example of the utility of PTRA is presented in
Figures 29.9,
29.10,
29.11,
29.12,
29.13,
29.14,
29.15,
29.16,
29.17 and
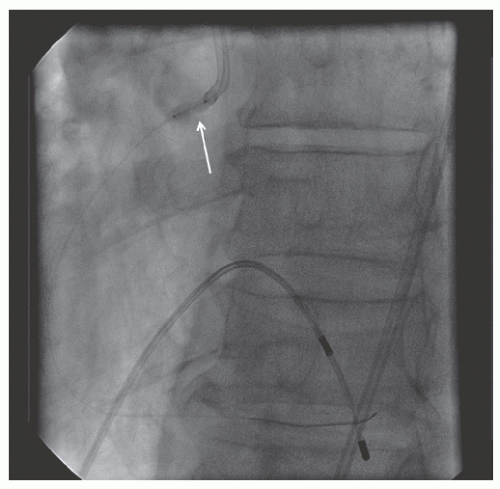
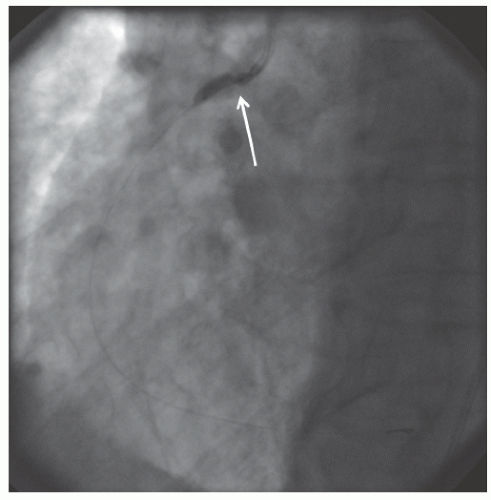
29.18. Rotablator should be avoided if there is angiographic evidence of dissection, thrombus, slow flow or no-reflow (
Figure 29.17), excessive vessel tortuosity, or severe left ventricular dysfunction. When aggressive balloon angioplasty has failed to dilate a lesion, PTRA at the same setting should only be considered with caution (
Figures 29.9,
29.10 and
29.11). If balloon-generated dissection is present, PTRA could compound the dissection or induce perforation. Rotational atherectomy has been used to pass through a stent cell to revascularize an ostial lesion in a jailed side branch. However, it is recommended that this approach be used only if the stent cell has been previously dilated; and a small burr is recommended, given that serious complications have been encountered when the burr could not be retracted through the stent.
Limitations and Complications of Rotational Atherectomy
Limitations of PTRA include high cost and lack of confirmed impact on restenosis. Procedural success is highly dependent on the operator’s technique and experience. Particularly in longer lesions, there is still a significant incidence of non-Q-wave myocardial infarction and no-reflow
29 related to particle embolization, spasm, or microactivation caused by burr surface velocity. In addition, adenosine released secondary to microactivation and red cell hemolysis may lead to bradycardia and atrioventricular block. Therefore, it is recommended that temporary venous pacing be used or have femoral access available during PTRA.