Aspiration-Related Pulmonary Disorders
Pneumonia—“Captain of the Men of Death”
—William Osler1
Aspiration is defined as the misdirection of oropharyngeal or gastric contents into the larynx and lower respiratory tract.2 An assortment of pulmonary syndromes may occur following aspiration depending on the quantity and nature of the aspirated material, the chronicity of aspiration, as well as the nature of the host’s defense mechanisms and the host’s response to the aspirated material. The most important syndromes include “aspiration pneumonitis” or Mendelson’s syndrome, which is a chemical pneumonitis caused by the aspiration of gastric contents, and “aspiration pneumonia,” an infectious process caused by the aspiration of oropharyngeal secretions colonized by pathogenic bacteria.2 While there is some overlap between these two syndromes they are distinct clinical entities. In addition, a variety of pulmonary conditions have been described from chronic recurrent occult aspiration, most notably “diffuse aspiration bronchiolitis.”3 Other aspiration syndromes include airway obstruction, lung abscess, exogenous lipoid pneumonia, chronic interstitial fibrosis, and Mycobacterium fortuitum pneumonia. This chapter will focus on the pathophysiology, clinical features and management of aspiration pneumonitis, aspiration pneumonia, and diffuse aspiration bronchiolitis.
ASPIRATION PNEUMONITIS
Aspiration pneumonitis is best defined as acute lung injury following the aspiration of regurgitated gastric contents.2 This syndrome occurs in patients with a marked disturbance of consciousness such as drug overdose, seizures, coma due to acute neurologic insults, massive cerebrovascular accident, following head trauma and during anesthesia. It is important to emphasize that aspiration pneumonitis only occurs in patients who have a depressed level of consciousness with impairment of airway protective reflexes. In clinical practice, drug overdose is the most common cause of aspiration pneumonitis, occurring in approximately 10% of patients hospitalized following a drug overdosage. Adnet and Baud4 demonstrated that the risk of aspiration increases with the degree of impairment in consciousness (as measured by the Glasgow Coma Scale). Historically, the syndrome most commonly associated with aspiration pneumonitis is Mendelson’s syndrome, reported in 1946 in obstetric patients who aspirated while receiving general anesthesia.5 Mendelson’s original report consisted of 44,016 nonfasted obstetric patients whom he studied between 1932 and 1945, of whom more than half received an “operative intervention” with ether by mask without endotracheal intubation. He described aspiration in 66 patients (1:667). Although several of the patients were critically ill from their aspiration, recovery was usually complete within 24 to 36 hours and only two patients died (1:22,008).
Although aspiration is a widely feared complication of general anesthesia, clinically apparent aspiration in modern anesthesia practice is exceptionally rare, and in healthy patients the overall morbidity and mortality are low (see section below). The risk of aspiration is greatly increased in patients intubated emergently in the field, emergency room or in the ICU. In these patients every effort should be made to reduce the risk of aspiration; this includes removing dentures and clearing the airway and in certain circumstances placing a nasogastric tube to empty the stomach prior to intubation. If there is an immediate risk of airway compromise endotracheal intubation should be performed prior to placement of a nasogastric tube. However, if the patient is likely to have a full stomach (upper GI bleed, small bowel obstruction, ileus, etc.) it may be prudent to place a nasogastric tube prior to endotracheal intubation. When intubating emergently, suction equipment must be immediately available and rapid-sequence induction using cricoid pressure should be performed.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Mendelson emphasized the importance of acid when he showed that unneutralized gastric contents introduced into the lungs of rabbits caused severe pneumonitis indistinguishable from that caused by an equal amount of 0.1 N hydrochloric acid.5–7 However, if the pH of the vomitus was neutralized before aspiration, the pulmonary injury was minimal. Experimental studies have demonstrated that the severity of lung injury increases significantly with the volume of the aspirate and inversely with its pH, with a pH of less than 2.5 being required to cause aspiration pneumonitis. However, the stomach contains a variety of other substance in addition to acid. Several experimental studies have revealed that aspiration of small, particulate food matter from the stomach may cause severe pulmonary damage, even if the pH of the aspirate is above 2.5.8,9 These studies suggest that cell recruitment and expression of inflammatory mediators are most pronounced after injury with combined acid and small food particles. These data are supported by findings in patients where the most severe lung injury was observed in patients following aspiration with particulate food matter.10,11
Aspiration of gastric contents results in a chemical burn of the tracheobronchial tree and pulmonary parenchyma with an intense parenchymal inflammatory reaction. The proinflammatory cytokines including tumor necrosis factor-α and CXC chemokines are crucial to the development of aspiration pneumonitis by mediating neutrophil recruitment. Once localized to the lung, neutrophils play a key role in the development of lung injury through the release of oxygen radicals and proteases. Gastric acid prevents the growth of bacteria and therefore the contents of the stomach are normally sterile. Bacterial infection, therefore, does not play a significant role in the early stages of acute lung injury following aspiration of gastric contents. However, acid aspiration pneumonitis reduces host defenses against infection increasing the risk of superinfection.12 The incidence of this complication has, however, not been well studied. Furthermore, experimental models suggest that acid aspiration pneumonitis “primes the lung” making secondary infection more severe.12,13 Colonization of the gastric contents by potentially pathogenic organisms may occur when the gastric pH is increased by the use of antacids, histamine-2 (H2) receptor blockers, or proton pump inhibitors. In addition, gastric colonization by gram-negative bacteria occurs in patients receiving enteral feedings, as well as in patients with gastroparesis and small bowel obstruction. In these circumstances the pulmonary inflammatory response is likely to result from both bacterial infection and the inflammatory response of the gastric particulate matter.
 ANESTHESIA AND ASPIRATION PNEUMONIA
ANESTHESIA AND ASPIRATION PNEUMONIA
Aspiration pneumonitis has traditionally been regarded as the major cause of serious anesthetic complications. However, with the recognition of the importance of this complication, and the fact that it is largely preventable, the risk of aspiration pneumonitis in modern anesthesia is very low. Nevertheless, aspiration pneumonitis is an important perioperative complication and remains the commonest cause of anesthesia-related death. The risk of aspiration with modern anesthesia is reported to be between 2.9 and 4.7 per 10,000 general anesthetics (about 1 in 3000 anesthetics) with a mortality of approximately 1:125,000, accounting for between 10% and 30% of all anesthetic deaths.14,15 Warner et al.7 published data from a study of 215,488 anesthetics and observed 67 episodes of aspiration in adults (3.1 per 10,000 patients) undergoing general anesthesia. In a recent study involving 99,441 patients undergoing nonobstetrical anesthesia, perioperative pulmonary aspiration occurred in 14 patients (1 in 7103 procedures).16 All 14 patients has one or more risk factors for aspiration. The Thai Anesthesia Incident Monitoring Study prospectively recorded reports on aspiration from 51 hospitals across Thailand during a 6-month period in 2007.17 Twenty-eight reports met the definition of pulmonary aspiration (denominator not reported). Most of the incidents occurred in American Society of Anesthesiology (ASA) class 1 to 2 patients (85.7%), during day time hours (64.3%), and when the anesthesiologists were in charge (67.9%). Eleven incidents (39.3%) occurred during induction, seven (25%) during maintenance, and seven (25%) during the emergence phase. All the incidents except one (96.4%) were considered human error and 25 (89.2%) were preventable. Thirteen patients (46.4%) had major physiologic changes and 10 (35.7%) of them required unplanned ICU admission. Ten patients (35.7%) needed prolonged ventilator support and two (7.14%) of them died.
The most definitive and extensive review on the risk of airway problems associated with intubation was reported by Cook et al.18,19 who summarized the findings of the 4th National Audit Project of the Royal College of Anaesthetists. In this 1-year prospective audit, the authors examined the occurrence of serious airway complications in anesthesia, ICUs, and emergency departments of all the National Health Service hospitals in the United Kingdom. The authors found 184 cases that met the inclusion criteria: 133 from anesthesia, 36 from ICUs, and 15 from emergency departments. A concurrent national census of anesthesia activity over a 2-week period was performed to provide denominator data for anesthesia, which indicated 2.9 million anesthetic procedures annually, resulting in a nominal incidence (using the 133 anesthesia events) of one serious airway complication per 22,000 anesthetics. In total, 38 deaths were attributable to airway complications, and the death rate was 16 of 133 (12%) in anesthesia, 18 of 36 (50%) in the ICUs, and 4 of 16 (25%) in the emergency departments. Pulmonary aspiration of gastric contents was the commonest cause of death and brain damage. A supraglottic airway was the planned technique in more than 50% of death or brain damage cases. A statistical analysis of the reports suggested that as few as 25% of relevant incidents may have been reported. The authors therefore suggest that this audit provides an indication of the lower limit for the incidence of such complications.
Emergency surgery (particularly, trauma and abdominal surgery with delayed gastric emptying) procedures performed at night, inadequate anesthesia, obesity, elderly immobilized patients, and obstructive sleep apnea have been associated with a higher risk of aspiration.15,20 Obtunded adults and children with known gastroesophageal reflux are more likely to aspirate even without narcotic or sedative impairment of airway reflexes. The factors increasing the risk for aspiration are listed in Table 69-1. Patients who are at risk before surgery are also at increased risk during the postoperative period when immobility, residual effects of anesthetic agents, and narcotics combine to decrease protective airway reflexes.21–23
The incidence of aspiration appears higher in obstetric patients as highlighted by the pioneering study of Mendelsohn.5 A study from 1973 reported an incidence of 1 in 6000 obstetric patients receiving general anesthesia for vaginal deliveries and 1 in 430 for cesarean section patients.24 In more recent studies, an aspiration incidence of 1 in 1547 and 1 in 1431, respectively, were described for cesarean section under general anesthesia,21,23 and a recent audit showed an incidence of 1 in 900 women undergoing cesarean section.22 The risk of pulmonary aspiration is, therefore, at least double or three times as high as in general surgical patients. Pregnant women are at increased risk of aspiration because of gastroesophageal reflux and delay in gastric emptying.25,26 Gastroesophageal reflux is common in pregnancy and can be demonstrated even in the absence of symptoms. There is no difference in basal and evoked gastric acid secretion in pregnancy but there is a reduction in lower esophageal barrier pressure, which is likely to be a progesterone effect present from early pregnancy. The surgical procedure may itself increase aspiration risk through the adoption of lithotomy or Trendelenburg positions or the creation of a pneumoperitoneum.
The laryngeal mask airway (LMA) does not reliably protect the lungs from regurgitated stomach contents and should be avoided in patients at an increased risk for aspiration.27,28 In a meta-analysis of 12,901 low-risk cases, where the standard contraindications to the use of the LMA were followed (e.g., absence of gastrointestinal pathology, obesity, history of reflux, or emergency surgery), only 3 cases of aspiration were identified, an incidence of 2.3 per 10,000.29
Inadequate reversal of neuromuscular blockage at the end of surgery is an important risk factor for aspiration. After the administration of nondepolarizing neuromuscular blocking agents, it is essential to ensure adequate return of normal neuromuscular function. Residual paralysis decreases upper esophageal tone, coordination of the esophageal musculature during swallowing, and the hypoxic ventilatory drive.30 These factors significantly increase the risk for aspiration. Adequate recovery of postoperative neuromuscular function cannot be guaranteed without objective neuromuscular monitoring. Good evidence-based practice dictates that clinicians should always quantitate the extent of neuromuscular blockade by objective monitoring (train-of-four monitoring).30 To exclude clinically significant residual neuromuscular blockade, the train-of-four ratio, when measured mechanically or by electromyography, must exceed 0.9. If sufficient recovery (i.e., train-of-four ≥0.9) has not been documented objectively at the end of the surgical procedure, the neuromuscular block should be antagonized.
Prevention of Aspiration During Anesthesia
In recent years more liberal preoperative fasting guidelines have been promoted. In healthy adults without an increased risk of regurgitation or aspiration solids should be avoided after midnight; however, a light meal such as dry toast may be considered up to 6 hours before anesthesia and clear liquids such as water, coffee without milk, or fruit juice can be given up to 2 hours before induction.31,32 Meta-analyses of randomized controlled trials comparing fasting times of 2 to 4 hours versus more than 4 hours report smaller gastric volumes and higher gastric pH values in adult patients given clear liquids 2 to 4 hours before a procedure and this approach is currently endorsed by the ASA.33
Preoperative antacids, H2 receptor blockers, proton pump inhibitors, and prokinetic agents have been used to reduce the volume and/or acidity of the gastric contents. There is, however, a lack of data indicating that any of these drugs reduce the risk of aspiration pneumonitis.34 The routine use of these drugs is not recommended by the ASA guidelines.33 However, it is not unreasonable to use these drugs in patients at an increased risk of aspiration. It is however important to realize that it takes time for the clinical effects of the acid suppressive drugs to manifest and these drugs should therefore be dosed at least 2 hours prior to induction.35
Standard teaching suggests that rapid-sequence induction with cricoid pressure should be performed when intubating patients at an increased risk of aspiration. Cricoid pressure, known as the Sellick maneuver, is designed to occlude the cervical esophagus by compressing it between the cricoid cartilage and the vertebral bodies.36 Passively regurgitated gastric contents are therefore prevented from entering the pharynx. Neither rapid-sequence induction nor cricoid pressure has been prospectively studied and proven to decrease the incidence of aspiration. Rapid-sequence induction shortens the time between the onset of unconsciousness and securing the airway, which may be of benefit if the aspiration risk is high. However, the use of cricoid pressure is controversial.37 The esophagus cannot be reliably occluded between the cricoid cartilage and the vertebral bodies. Studies in volunteers (men and nonpregnant women) clearly show that the esophagus often does not lie in the midline or that cricoid pressure displaces the esophagus laterally without occluding it.38 More recent data suggest that the hypopharynx posterior to the cricoid cartilage is in continuity with the esophageal inlet and this hypopharyngeal area is occluded with the Sellick maneuver.39 Cricoid pressure is often applied incorrectly or without the appropriate amount of force and may reduce visualization of the vocal cords and impede intubation. Although not proven to reduce the risk of aspiration during emergent intubations, cricoid pressure is currently considered the standard of care in this situation.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
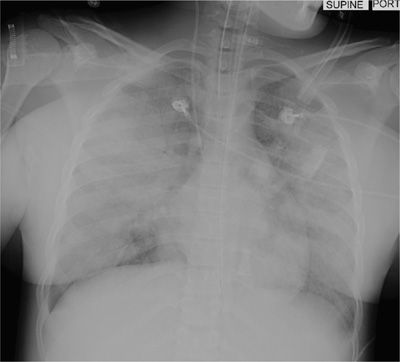
Aspiration of gastric contents can present dramatically with a full-blown picture that includes gastric contents in the oropharynx, wheezing, coughing, shortness of breath, cyanosis, pulmonary edema, hypotension, and hypoxemia; this may progress rapidly to severe ARDS and death (see Fig. 69-1). However, many patients may not develop signs or symptoms associated with aspiration, while others may develop a cough or wheeze. In some patients aspiration may be clinically silent manifesting only as arterial desaturation with radiologic evidence of aspiration. Warner et al.7 studied 67 patients who aspirated while undergoing anesthesia. Forty-two (64%) of these patients were totally asymptomatic, 13 required mechanical ventilatory support for more than 6 hours and 4 died.
Figure 69-1 Anteroposterior chest radiograph demonstrating bilateral alveolar infiltrates following aspiration of gastric contents (aspiration pneumonitis).
 MANAGEMENT OF ASPIRATION PNEUMONITIS
MANAGEMENT OF ASPIRATION PNEUMONITIS
The upper airway should be suctioned following a witnessed aspiration. Endotracheal intubation should be considered in patients who are unable to protect their airway. While common practice, the prophylactic use of antibiotics in patients with suspected or witnessed aspiration is not recommended. Similarly, the use of antibiotics shortly after an aspiration episode in a patient who develops a fever, leukocytosis, and a pulmonary infiltrate is discouraged as it may select for more resistant organisms in a patient with an uncomplicated chemical pneumonitis. However, empiric antimicrobial therapy is appropriate in patients who aspirate gastric contents in the setting of small bowel obstruction or in other circumstances associated with colonization of gastric contents (acid-suppressive therapy, tube feeds). Antimicrobial therapy should be considered in patients with an aspiration pneumonitis that fails to resolve within 48 hours. Empiric therapy with broad-spectrum agents is recommended. Antimicrobials with anaerobic activity are not routinely required. Recently serum procalcitonin has emerged as a biomarker that has been postulated to be able to discriminate bacterial infections from nonbacterial inflammatory disorders.40 El-Solh et al.41 investigated the predictive accuracy of serum procalcitonin in distinguishing aspiration with bacterial pneumonia from “sterile” aspiration pneumonitis. In this study serum procalcitonin levels had poor diagnostic value in separating bacterial pneumonia from aspiration pneumonitis based on quantitative bronchoalveolar lavage culture.
 IMMUNOMODULATING AGENTS
IMMUNOMODULATING AGENTS
Corticosteroids have been used in the management of aspiration pneumonitis since 1955.42 However, limited data exist on which to evaluate the role of these agents, with only a single prospective, placebo-controlled study having been performed. In this study, Sukumaran et al.43 randomized 60 patients with “aspiration pneumonitis” to methylprednisolone (15 mg/kg/day for 3 days) or placebo. The patients were subdivided into two groups; a younger group with drug overdose as the predominant diagnosis and an older group with neurologic disorders. Radiographic abnormalities improved more rapidly in the steroid group, as did oxygenation. The number of ventilator and ICU days was significantly shorter in the overdose patients who received corticosteroids; however, these variables were longer in the neurologic group receiving this therapy. There was no significant difference in the incidence of complications or mortality. The results of this study are somewhat difficult to interpret as it is likely that the patients in the overdose group had true “aspiration pneumonitis” while many patients in the neurologic group probably developed “aspiration pneumonia.” Wolfe et al.44 performed a case-controlled study of 43 patients with aspiration pneumonitis, of whom 25 received high-dose corticosteroids (approximately 600 mg prednisolone/day for 4 days). While there was no difference in mortality, secondary gram-negative pneumonia was reported to be more frequent in the steroid group (7/20 vs. 0/13); however, ventilator days tended to be less in this group (4.3 vs. 9.8 days). Based on this limited data it is not possible to make evidence-based recommendations on the use of corticosteroids in patients with aspiration pneumonitis.
In animal models, a number of pharmacologic interventions including inhaled B2 agonists, pentoxifylline, antiplatelet drugs, and omega-3 fatty acids have been shown to attenuate the acute lung injury following acid aspiration.45–50 The role of these interventions in patients remains to be tested; however, due to their inherent safety, these agents should be considered in patients with severe acid aspiration pneumonitis.
ASPIRATION PNEUMONIA
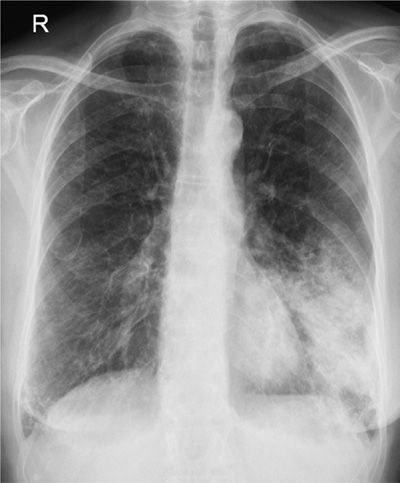
Aspiration pneumonia refers to the development of a radiographic infiltrate and clinical features consistent with pneumonia in a patient with risk factors for increased oropharyngeal aspiration (see Fig. 69-2). Approximately half of all healthy adults aspirate small amounts of oropharyngeal secretions during sleep. Presumably, the low virulent bacterial burden of normal pharyngeal secretions together with forceful coughing, active ciliary transport, and normal humoral and cellular immune mechanisms result in clearance of the inoculum, without sequelae. However, if the mechanical, humoral, or cellular mechanisms are impaired or if the aspirated inoculum is large enough, pneumonia may follow. Any condition that increases the volume and/or bacterial burden of oropharyngeal secretions in the setting of impaired host defense mechanism may lead to aspiration pneumonia. Indeed, in stroke patients undergoing swallow evaluation there is a strong correlation between the volume of the aspirate and the development of pneumonia.51 Factors that increase oropharyngeal colonization with potentially pathogenic organisms and that increase the bacterial load may augment the risk of aspiration pneumonia. The clinical setting in which pneumonia develops largely distinguishes aspiration pneumonia from other forms of pneumonia. However, there is much overlap. This is illustrated by the fact that otherwise healthy elderly patients with “community-acquired pneumonia” (CAP) have been demonstrated to have a significantly higher incidence of silent aspiration when compared with age-matched controls.52
Figure 69-2 Anteroposterior chest radiograph demonstrating a left lower lobe aspiration pneumonia.
 EPIDEMIOLOGY
EPIDEMIOLOGY
The lack of specific and sensitive markers of aspiration makes the epidemiologic study of aspiration syndromes difficult. Furthermore, most studies do not make the distinction between aspiration pneumonitis and aspiration pneumonia. Nevertheless, several studies list “aspiration pneumonia” as the cause of CAP in 5% to 15% of cases.53,54 CAP is a major cause of morbidity and mortality in the elderly and it is likely that aspiration is the major cause of pneumonia in these patients. Epidemiologic studies have demonstrated that the incidence of pneumonia increases with aging, with the risk being almost six times higher in those over the age of 75, compared to those less than 60 years of age.55,56 The attack rate for pneumonia is highest among those in nursing homes.57
 DYSPHAGIA IN PATIENTS WITH ASPIRATION PNEUMONIA
DYSPHAGIA IN PATIENTS WITH ASPIRATION PNEUMONIA
Swallowing is a complex function, with both voluntary and reflexive components. Five cranial nerves and more than 50 muscles in the head and neck are involved in oropharyngeal swallowing. Both brainstem and cortical areas are involved in the neural processing of swallowing. The coordination of swallowing requires bilateral input from the sensorimotor cortex with descending input to the brainstem medullary swallowing center.58 Functional and anatomic imaging studies have identified several sites that play an important role in swallowing, including the primary sensorimotor cortex, insula, anterior cingulate, internal capsule, basal ganglia, and thalamus. The swallowing process can be divided into oropharyngeal phase and esophageal phase.59 The oropharyngeal phase includes biting and chewing in the oral cavity, and the transport of food into the pharynx. In simplified terms, this process is accompanied by elevation and anterior movement of the larynx to meet with the epiglottis for protection of the airway. It is followed by passage of the bolus through the upper esophageal sphincter into the esophagus (esophageal phase). During the esophageal phase, the lower esophageal sphincter relaxes and food is pushed into the stomach by peristalsis and gravity.
Dysphagia refers to the difficulty in swallowing. The severity of dysphagia varies from moderate difficulty to complete inability to swallow. Dysphagia is the major risk factor leading to aspiration pneumonia. In addition, dysphagia contributes significantly to protein-energy malnutrition and dehydration. Impairment in any component of the swallow mechanism including anatomical abnormities of the upper airway or esophagus can lead to dysphagia. Dysphagia has traditionally been associated with brainstem and bilateral cerebral infarction, though it has more recently been shown to occur in isolated cerebral infarctions as well. Furthermore, dysphagia is commonly associated with silent cerebral infarction.
Dysphagia is remarkably common in Westernized nations and is a major cause of morbidity and mortality. Indeed, aspiration pneumonia is probably the final common pathway by which most chronically ill patients die. It has been estimated that over 16 million senior citizens in the United States suffer from dysphagia.60 Furthermore, an additional 300,000 to 600,000 patients develop dysphagia each year in the United States from neurologic disorders.61 Dysphagia affects more than 30% of patients who have had a cerebrovascular accident; 52% to 82% of patients with Parkinson disease; 84% of patients with Alzheimer disease, up to 40% adults aged 65 years and older, and more than 60% of elderly institutionalized patients.62 The efficiency of the swallow mechanism decreases with aging, increasing the risk of aspiration and pneumonia in the elderly. Kikuchi et al. evaluated the occurrence of silent aspiration in otherwise “healthy elderly patients” with CAP and age-matched control subjects using indium111 chloride scanning.52 Silent aspiration was demonstrated in 71% of patients with CAP compared to 10% in control subjects. The impaired swallow mechanism in the elderly can be attributed to diminished sensation, silent cerebral infarction, cerebral atrophy, a delay in the synapse conduction in the afferent inputs to the central nervous system, and lingual weakness (sarcopenia) caused by aging.63,64
 RISK FACTOR FOR DYSPHAGIA
RISK FACTOR FOR DYSPHAGIA
The major risk factors for dysphagia are listed in Table 69-2. In patients with an acute stroke the incidence of dysphagia ranges from 40% to 70%.65 Dysphagic patients who aspirate are at an increased risk of developing pneumonia.66,67 Although dysphagia improves in most patients following a stroke, in many the swallowing difficulties follow a fluctuating course with 10% to 30% continuing to have dysphagia with aspiration.68,69