Reducing the risk of stroke during carotid artery stenting requires a combination of proper patient selection and impeccable technique. Each phase of the procedure carries risk, and achieving optimal outcomes involves meticulous attention to detail at each step. It is essential for the interventionalist to have a thorough understanding of cerebrovascular anatomy, vascular biology, and anatomic variations, along with a complete knowledge of the full spectrum of available equipment.
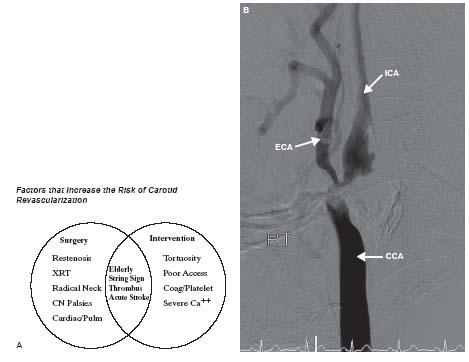
The importance of proper patient selection cannot be overstated. A number of patient characteristics increase the risk of either surgical or percutaneous carotid revascularization (Fig. 11.1). Patients with visible thrombus in the internal carotid artery (ICA) should be avoided and referred for surgical revascularization. However, if absolutely necessary, these patients can be addressed percutaneously with proximal occlusion systems by experienced operators. Similarly, if tortuosity of the ICA precludes the use of distal embolic protection devices (EPDs) (see below), the patient should be referred for endarterectomy unless the operator is comfortable with the use of proximal occlusions systems.
There are several steps during carotid stenting that must be successfully negotiated to achieve procedural success. Figure 11.2 shows the risk of stroke associated with the three main phases of the procedure. Data from the XACT, CAPTURE, and CASES PMS (1,2) postmarket registries indicate that at 30 days, about 10% to 15% of strokes have occurred in the contralateral hemisphere. This suggests that these strokes are due to diagnostic catheter manipulation during angiography or subsequent sheath or guide placement in the common carotid artery (CCA). Assuming that an equal number of strokes that occur in the ipsilateral hemisphere are also due to catheter manipulation, it is likely that about 20% to 30% of strokes at 30 days are likely due to catheter manipulation in the carotid. This number may be slightly inflated as it includes strokes that occurred after discharge that are unrelated to carotid artery disease.
Analyses of data from the CAPTURE registry suggest that approximately 20% of strokes occur post-procedure (2). This figure is questionable, however, given the timing of neurologic follow-up post-procedure. Minor peri-procedural strokes are often missed by operators and not detected until the next day when the patient is seen by the neurologist. Thus, these strokes may be misclassified as post-procedural strokes. It is the authors’ experience that a small minority of strokes occur postprocedure (probably less than 5%).
The majority of strokes occur at the time of the stenting procedure itself (Fig. 11.3). Transcranial Doppler (TCD) data from multiple studies have shown that there are microemboli to the middle cerebral artery (MCA) during all phases of the procedure (3). However, the risk of stroke is not equal in all phases of the procedure. Among the three phases of the procedure, only postdilation has been shown to be an independent predictor of stroke (4).
ANGIOGRAPHY
Reducing the risk of stroke during carotid artery stenting begins with adhering to meticulous technique during diagnostic angiography. There are a few basic tenets that can reduce the risk of stroke with each individual step, thereby contributing to a significantly lower overall risk for the procedure (Table 11.1).
Use of a manifold allows real-time monitoring of hemodynamics throughout the case, and we believe that it is essential to use a manifold during carotid artery stenting. This is especially important in patients with concomitant coronary artery disease or aortic stenosis. It also helps reduce the risk of injecting into plaque by monitoring for dampening of waveforms during catheter positioning.
It is also important not to power inject into the common carotid arteries. In situations where power injection is being used, the physician often leaves the patient’s bedside after positioning the diagnostic catheter in the CCA. Patient movement during this time can result in inadvertent angulation of the diagnostic catheter toward the vessel wall, and subsequent power injection can result in carotid dissection. Instead, the authors recommend hand injections with routine use of a manifold. This minimizes the risk of air embolism associated with direct connection of contrast-filled syringes, and also reduces the risk of complications related to power injection.
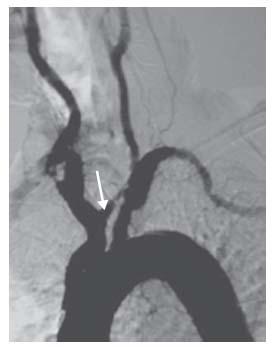
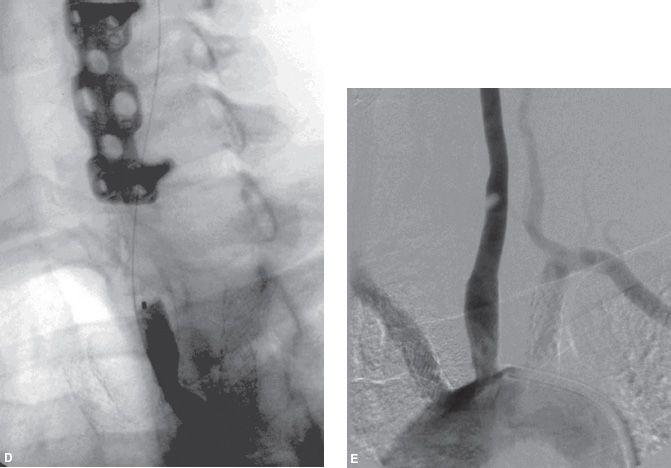
The vertebral arteries should not be routinely cannulated during carotid angiography, and selective vertebral artery angiography should be reserved for those cases where knowledge of posterior circulation anatomy will help with decision making, or where there is a true concern for vertebrobasilar insufficiency. In such cases, nonselective injection of contrast into the subclavian arteries, while a blood pressure cuff is inflated on the ipsilateral arm, may suffice. If cannulation is truly indicated, then the ostium of the vertebral artery must be visualized prior to selection with a diagnostic catheter as ostial disease of the vertebral arteries is quite common (Fig. 11.4). The contralateral oblique projection is usually best for optimal visualization of the vertebral artery ostium.
Figure 11.1 • A: Factors that increase the risk of carotid revascularization. XRT, radiation therapy; CN, cranial nerve; Pulm, pulmonary; Coag, coagulopathy; Ca++, calcification. B: Example of carotid stenosis that is high-risk for stenting due to severe calcification of the distal common carotid artery and proximal internal carotid artery. Such severe concentric calcification can preclude optimal stent expansion and there is also a risk of carotid perforation if excessive post-dilatation is performed to try to achieve adequate stent expansion. CCA, common carotid artery; ICA, internal carotid artery; ECA, external carotid artery.
SEVERE TYPE III AORTIC ARCH ANATOMY
The approach to carotid stenting based on arch type has been described in Chapter 10. However, in some patients the angle of origin of the innominate artery or the left CCA is so acute that attempts to advance wires into the CCA or external carotid artery (ECA) through a regular or reverse curve diagnostic catheter result in the catheter being prolapsed into the aorta (Fig. 11.5A, B).
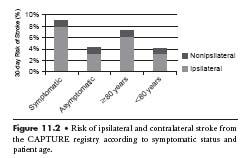
Figure 11.2 • Risk of ipsilateral and contralateral stroke from the CAPTURE registry according to symptomatic status and patient age.
In such cases, the authors recommend using a modification of the telescoping technique. A H1 guide catheter is used to directly engage the innominate artery or the left CCA, as shown in Figure 11.5C. Care must be taken to minimize manipulation of the guide in the aortic arch. Once the guide is in position, a long 0.035″ glidewire is carefully advanced into the ECA under roadmap guidance. A 5 Fr. 125-cm diagnostic catheter, such as a JR4, is then advanced into the ECA over the glidewire through the H1 guide. Once in position, the H1 guide can be telescoped over the diagnostic catheter and glidewire into position. Care must be taken to monitor for sudden forward movement of the entire system as the guide is advanced into the CCA, as this can result in plaque dissection or embolization.
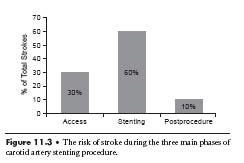
Figure 11.3 • The risk of stroke during the three main phases of carotid artery stenting procedure.
Another option is to use a Simmons guide catheter to access the innominate or left CCA. The Simmons catheter can be shaped in the descending thoracic aorta, ascending thoracic aorta, or using the left subclavian artery. Usually a Simmons 1 catheter will not be long enough, and a Simmons 2 will work better (5). However, care must be taken as excessive manipulation of this catheter in the aortic arch can increase the risk of stroke, especially in older patients.
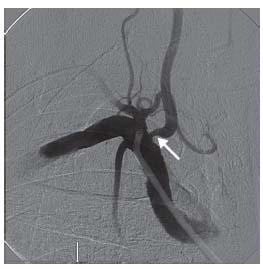
Figure 11.4 • Moderate ostial stenosis of right vertebral artery (arrow).
A bovine origin of the left CCA combined with a steep type III arch can be particularly challenging. In such cases, both the Vitek and Simmons 2 diagnostic catheters are useful options, particularly if combined with the telescoping technique. Alternatively, an AL 1 guide catheter can be used to engage the proximal left CCA, and can remain in this position during the procedure. This is also a useful technique in patients with proximal left CCA disease with a bovine aortic arch (Fig. 11.6). When using guides that engage only the proximal part of the carotid, a stiff 0.014″ coronary buddy wire should be positioned in the ECA for support because there is a risk of guide prolapse during stent advancement; guide or sheath prolapse while an EPD is deployed can result in the open EPD being pulled through the lesion with adverse consequences. Guide and sheath position should be monitored during balloon and stent advancement by either panning or using a large field of view.
OSTIAL INNOMINATE ARTERY AND LEFT COMMON CAROTID ARTERY DISEASE
Ostial lesions of the innominate artery and left CCA are challenging to treat, but with the proper technique, can be treated safely and effectively percutaneously. These are complicated cases with multiple steps, and should only be treated by an experienced operator. The key tenet is that the guide or sheath must not cross or touch the lesion at any point, and must remain in the aorta at all times. Patients may present with combined internal carotid and ostial CCA or innominate disease, and both can be treated in the same setting. In these cases, the ICA must be stented before the ostial CCA as it may be difficult to advance a self-expanding stent through a deployed ostial stent. Ostial disease is often calcified and recalcitrant to dilation, and it is therefore preferable to use balloonexpandable stents as compared to self-expanding stents. Balloon-expandable stents offer a further advantage of more precise placement and deployment, which is critical when treating ostial disease. Before beginning, it is important to start with a good quality aortic arch angiogram in the left anterior oblique (LAO) projection to properly evaluate the ostia of the great vessels. A step-by-step method of safely treating ostial lesions of the left CCA and right CCA is described below.
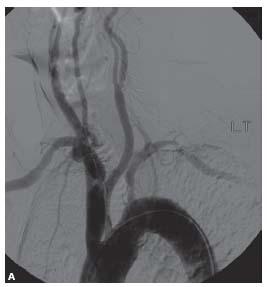
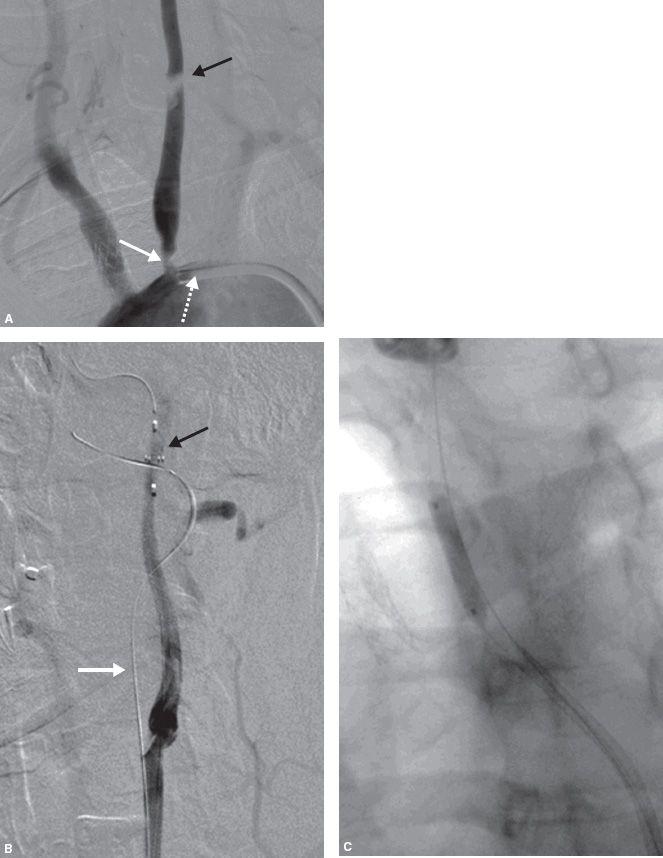
Figure 11.5 • Some patients have such acute angles of origin of the great vessels from the aortic arch (A) that advancing a 0.035″ wire into the vessel through a normal or reverse curve diagnostic catheter results in catheter prolapse (B).
Figure 11.5 • In such cases, it may be useful to cannulate the ostium of the innominate artery or left common carotid artery directly with an 8 Fr. H1 guide catheter, as shown in this RAO projection angiogram (C).
Ostial Left CCA Stenosis
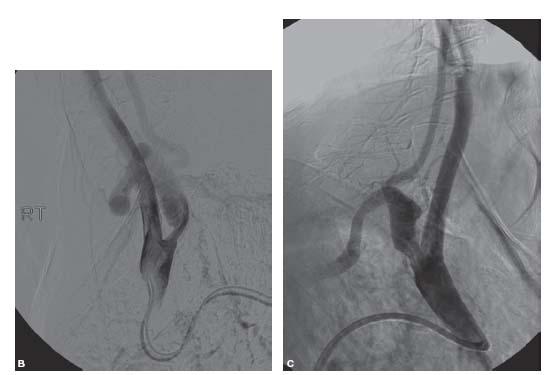
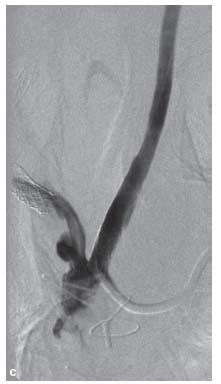
Moderate ostial disease that does not require treatment but is found incidentally in the presence of a severe internal carotid stenosis may be crossed with a diagnostic catheter or sheath. This is one instance in which we prefer a sheath to a guide due to the better transition between the sheath and the dilator that should cause less trauma to the ostial lesion (Fig. 11.7).
For severe lesions of the left carotid ostium that warrant treatment, a “no-touch” technique is highly recommended. The lesion should not be crossed or engaged with diagnostic or guide catheters; sheaths should not be used for these lesions. Guide shapes that will not accidentally engage the CCA should be chosen. Our preferred guide is the 8 Fr. Multipurpose, which can usually be placed adjacent to the ostium but will typically not engage the ostium (Fig. 11.8).
With the Multipurpose guide adjacent to the ostium, an extra-support 0.014″ wire (e.g., Grand Slam, Abbott Vascular, Menlo Park, CA) should be advanced across the lesion and placed in the ECA. If this proves to be excessively difficult, a Vitek catheter can be advanced through the guide and used to steer the wire across the lesion; if at all possible, the Vitek should not be advanced across the lesion. Once the 0.014″ wire is in the ECA, an EPD should be placed in the ICA. At this point, a 0.014″ compatible balloon is advanced over the EPD wire and the lesion is pre-dilated. A 0.014″ compatible balloon-expandable stent is then advanced over the EPD wire; 7- or 8-mm diameter stents are usually adequate and in smaller patients, 6 mm diameter stents may be adequate. These lesions are typically associated with significant aortic arch disease and calcification, and it is possible to cause dissections and perforations of the aortic arch with aggressive sizing. The approach to these lesions is much more conservative than that with coronary ostial disease. The 0.014″ buddy wire in the ECA should not be removed until the EPD is removed and the final angiogram taken. Sandwiching this wire between the stent and CCA is not a problem and it can easily be removed at the end. Hydrophilic wires should not be used as buddy wires because it is possible to strip the hydrophilic coating during wire removal.
Figure 11.6 • Bovine origin of the left common carotid artery (arrow) in a patient with a type III aortic arch. The acute angulation of the left common carotid artery origin makes carotid stenting from the femoral approach challenging. In such cases, right radial or brachial approaches may be useful. This patient has concomitant stenosis of the proximal left and right common carotid arteries, adding to the complexity of the case.
Ostial left CCA lesions with a bovine arch can be particularly challenging. These are best approached with an AL 1 guide; occasionally a H1 guide may be appropriate (Fig. 11.9). The remainder of the procedure is as described above. There is a tendency for the AL 1 guide to engage the left CCA and cross the lesion once the buddy wire is in place; mild forward pressure on the guide will prevent this from occurring.
Ostial Innominate Artery Disease
As with ostial left CCA disease, high-grade stenoses of the innominate artery are challenging to treat due to the location, short length, and highly variable diameter of the vessel, often due to post-stenotic dilation. Furthermore, these lesions are usually heavily calcified. The combination of these factors makes it difficult to find an appropriately sized stent in terms of length, diameter, and radial force. Balloon-expandable stents are most often the correct choice due to the shorter lengths that are available, ability to position precisely, and greater radial force. However, there is a higher risk of size mismatch and malapposition of portions of the stent. This increases the risk of stent migration and dislodgment, which can have disastrous consequences.
The technique for percutaneous revascularization of the innominate artery is similar to that of ostial left CCA stenoses. For true ostial lesions that are severe, every attempt should be made not to cross the lesion with either a diagnostic or guide catheter and the “no-touch” technique described for ostial left CCA disease should be employed.
For less critical lesions, it is permissible to cross the lesion with a 4 or 5 Fr. soft-tipped angled diagnostic catheter, such as a glide catheter or a Bernstein catheter. In cases of severe tortuosity, a JR4 or reverse curve catheter can be used. Ensure that the waveform is not dampened prior to any contrast injections. Use of a manifold is recommended to allow monitoring of hemodynamics throughout the case and prevent inadvertent injection into plaque. Under roadmap guidance, advance a 0.035″ glidewire into the right subclavian artery through the diagnostic catheter in the right anterior oblique (RAO) projection. A stiff glidewire may be used if needed. Advance the diagnostic catheter into the right subclavian artery, and remove the glidewire. Advance a long (i.e., 260 cm) Super Stiff Amplatz wire or equivalent into the subclavian artery through the diagnostic catheter, and then remove the diagnostic catheter. Remove the short sheath from the patient’s groin, and advance an 8 Fr. sheath over the Amplatz wire and position if in the aorta, with its tip adjacent to the ostium of the innominate artery. Because of the size of the stents needed to treat the innominate artery and the need for buddy wires, 8 Fr. sheaths are generally preferred. In particularly challenging cases, a 9 Fr. guide may be needed. Care must be taken not to disturb the lesion with the shuttle sheath or its dilator. Leaving the Amplatz wire in place, advance a 0.018″ guide-wire into the right subclavian artery, and then remove the Amplatz wire. Advance a 0.014″ filter–type EPD into the right ICA, and deploy it in a suitable position in the cervical segment. Pre-dilate the innominate artery lesion with a suitably sized balloon over the 0.014″ EPD wire. Take care to ensure that the balloon does not extend into the right CCA or right subclavian artery. Advance a suitably sized 0.014″ balloon-expandable stent over the EPD wire. If a 0.035″ balloon-expandable stent is used, it can be advanced over both the 0.018″ and 0.014″ wires. If it is truly an ostial lesion, then the stent will have to protrude slightly into the aorta to ensure proper coverage of the lesion. In patients with ostial disease, it is a good idea to post-dilate the ostial segment. Gently pull back the stent balloon, so that the distal portion is in the ostium, and post-dilate. The balloon should be pulled back very carefully as this can dislodge the stent. In patients with severe calcification, there is a risk of perforation with aggressive balloon dilation. If the patient complains of pain, stop inflating and deflate the balloon immediately. Once this step is complete, remove the EPD from the cervical ICA using the supplied recovery sheath, and then remove the 0.018″ buddy wire in the subclavian artery after performing final angiography.
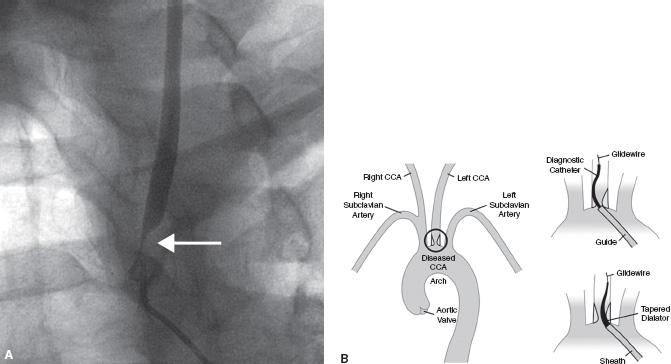
Figure 11.7 • A: Ostial left common carotid artery (CCA) stenosis (white arrow). This patient presented with crescendo left hemispheric transient ischemic attacks. Angiography was performed with a 5 Fr. Berenstein catheter (Boston Scientific, MA) positioned near the true ostium. B: Schematic illustration of management of moderate ostial left CCA stenosis in setting of treatment of internal carotid artery stenosis. Moderately diseased ostia can be crossed with a sheath; a sheath is preferable to a guide due to the better transition between the dilator and catheter resulting in less trauma to the plaque.
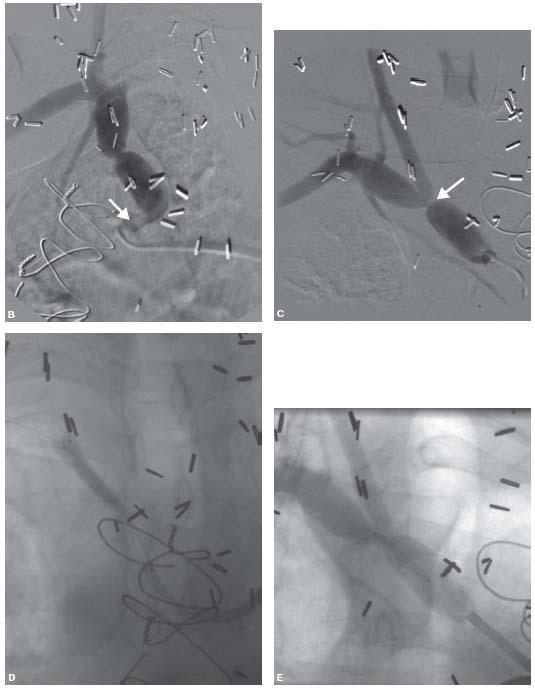
Figure 11.8 • A: Severe disease of the ostium (white arrow) and proximal (black arrow) left common carotid artery (CCA). An 8 Fr. Multipurpose (MP) guide has been positioned adjacent to the ostium (tip of guide indicated by interrupted white arrow). Note that the shape of the MP guide makes it unlikely that it will accidentally engage the left CCA. B: An extra-support wire (white arrow) has been placed in the external carotid artery (ECA) and a filter–type embolic protection device (EPD) (black arrow) has been placed in the internal carotid artery (ICA). C: Predilation over the EPD wire.
Figure 11.8 • D: Placement of balloon-expandable stent at ostium of left CCA over the EPD wire. Note that the buddy wire has been sandwiched between the stent and artery wall. The buddy wire should be removed at the end of the procedure after removal of the EPD. E: Final angiogram of the left CCA.
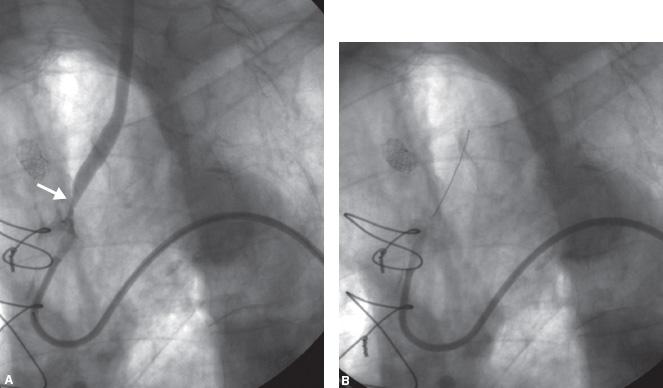
Figure 11.9 • A: Bovine origin of the left common carotid artery (CCA) with severe ostial disease (arrow). An AL 1 guide is positioned proximal to the lesion without engaging the artery. B: A 0.014″ extra-support wire is passed into the external carotid artery (ECA) prior to placement of embolic protection device (EPD) in the internal carotid artery (ICA).
Figure 11.9 • C: Final angiographic result following placement of balloon-expandable stent.
For innominate bifurcation lesions, a kissing balloon technique is necessary (Fig. 11.10). This generally necessitates the use of an 8 Fr. sheath or 9 Fr. guide. The CCA is much more important than the subclavian artery and should never be jailed in an attempt to obtain a perfect subclavian artery result; if absolutely necessary, balloon-expandable kissing stents may be placed with creation of a double-barrel innominate. The subclavian artery should be wired with a medium- or extra-support 0.014″ wire and the ICA with a 0.014″ filter–type EPD. Kissing balloon infl ations should be performed with conservatively sized balloons. If the tortuosity is not severe, a 0.035″ stent can be advanced over both wires and deployed at the bifurcation and then postdilated with kissing balloons. Alternatively, a 0.014″ stent can be deployed over the filter wire and the subclavian artery is rewired through the stent and final kissing balloon inflation performed. If there is concomitant ostial disease, a larger stent should be placed at the ostium and the stent balloon advanced into the previously placed distal stent and the overlap segment postdilated.
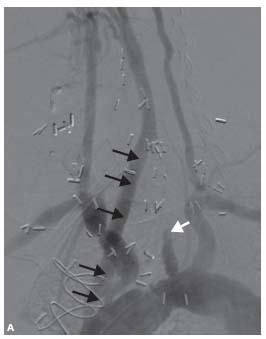
Figure 11.10 • A: A 50-year-old man reported right arm claudication and dizziness with upper extremity exertion. An 80-mm Hg pressure difference was noted between both arms. He was not deemed to be a surgical candidate due to the presence of severe three-vessel coronary allograft vasculopathy. Of note, he had a motor vehicle accident in 1992 resulting in occlusion of his left common carotid artery (CCA) (white arrow) requiring ascending aorta to left CCA bypass (black arrows).
Figure 11.10 • B: LAO view demonstrating a long tubular stenosis at the ostium of the innominate artery (arrow) with significant tortuosity. C: RAO view demonstrating a critical innominate artery bifurcation stenosis (arrow). D: Kissing balloon pre-dilation of innominate artery bifurcation with 3.0 mm diameter balloons. E: 8 X 18 mm balloon-expandable stent placed over both wires.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree