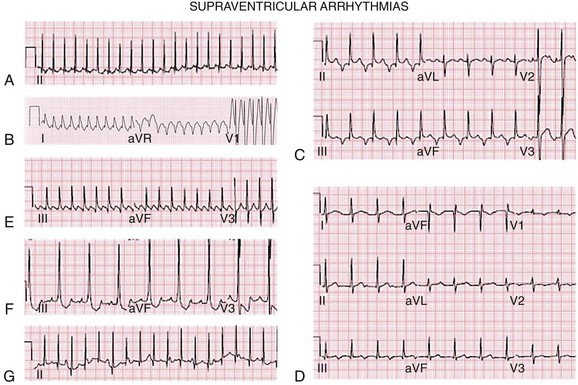
109 The correct interpretation and treatment of pediatric rhythms requires an understanding of the normal cardiac developmental variation that occurs as the child moves from fetal to neonatal life and then progresses toward adulthood. The current electrocardiogram (ECG) standards used in the United States were developed by Davignon in 19791 and only reflect differences by age. Rijnbeek et al.2,3 published normal standards for Dutch children, but they do not reflect racially and ethnically diverse populations. Appropriate assessment and management of arrhythmias in pediatric patients requires an understanding of the clinical presentation and the distinctive responses of children to the variety of available therapies. Premature ventricular contractions (PVCs) are relatively common in children, occurring in 1% to 2% of those with normal hearts and in as many as 50% to 60% of adolescents.4,5 PVCs may be seen in 15% of normal newborns and two thirds of adolescents and adults with repaired congenital heart disease.6 There is a marked difference in prognosis between PVCs in children with normal and abnormal hearts, thus investigation for associated conditions should be undertaken. Isolated PVCs are generally benign when there is no structural or functional heart disease, and they usually do not require treatment. Both of these arrhythmias can be seen in association with intake of stimulants including coffee, tea, and decongestants. Other associations include intercurrent illness and systemic illness such as hyperthyroidism, adrenal tumors (pheochromocytoma), or myocarditis, including that associated with Lyme disease. More complex ventricular arrhythmias including couplets, triplets, and nonsustained ventricular tachycardia (VT) may require further evaluation and treatment in some instances. Supraventricular tachycardia is a rapid rhythm that involves the atria, atrioventricular (AV) node, or accessory bypass tracts. It is the most common tachycardia seen in infants and children, with an incidence of 1 in 250 to 1000, occurring most commonly before 1 year of age. The other common developmental periods with increased SVT include approximately 5 to 7 years of age and during adolescence, both periods of rapid metabolic and developmental change. Patients can exhibit tachycardia during fetal development, with diagnosis suggested by auscultation of the infant’s heart rate during a prenatal examination and confirmed by fetal ultrasonography or magnetocardiography.7 Neonates and infants with tachycardia often exhibit signs and symptoms of congestive heart failure, which occurs if the tachycardia is present and not interrupted for more than 48 to 72 hours. The most common mechanism of SVT in children is AV reentry through an accessory pathway between the atrium and the ventricle or within the AV node; this represents 75% to 90% of arrhythmias in children without other cardiac conditions.8 Two thirds of these pathways are concealed (only unidirectional retrograde conduction) and one third are manifest as Wolff-Parkinson-White (WPW) or other forms of preexcitaiton. AV nodal reentry comprises 15% of pediatric SVT, more commonly seen in adolescents, with approximately one-third of SVT seen in adolescence being the result of AV nodal reentry (AVNRT).8 Automatic atrial ectopic tachycardia is the mechanism in 10% to 18% of SVT in children. Atrial flutter and atrial fibrillation represent 10% of nonpostoperative SVT and 40% of postoperative SVT; junctional ectopic tachycardia represents less than 1% of overall SVT. Although the majority of SVTs will resolve at around 1 to 2 years of age, one-third will recur by adolescence. Medications used to treat pediatric arrhythmias are shown in Box 109-1 and Table 109-1. Table 109-1 Agents for Chronic Treatment of Supraventricular and Ventricular Tachycardia Fifty to sixty percent of SVTs in children occur in the first year of life, with accessory pathway–mediated reentrant tachycardia (AVRT) being the most common mechanism.9 The percentage of patients with AVNRT increases with age. The heart rate in infants with SVT is usually 220 to 320 beats/min (bpm), with the heart rate in older children being in the 160 to 280 bpm range. The ECG may show either a narrow or a wide complex when it is aberrantly conducted or when antidromic SVT occurs with WPW (Figure 109-1, A and B). There are no trials to indicate the best drugs for SVT control, so individual or institutional preferences form the basis of treatment of many of these arrhythmias. Approximately one third of all patients in whom SVT develops within the first 3 months of life will outgrow it by 1 year of age.10 Recurrence is seen in one third of patients if a WPW pattern persists, but in only 10% of those with AVRT secondary to a concealed accessory pathway.11 Catheter ablation becomes a reasonable therapeutic option at approximately 4 to 5 years of age but is generally reserved for children older than 6 to 8 years in most institutions unless medications are not effective or life-threatening episodes, which are rare, occur. Ablation can and has been performed in younger children, but in patients younger than 4 years, the risks of the procedure increase.12 Recent experience has been recorded to perform these procedures with advanced mapping technology to minimize or eliminate the use of radiation.13,14 Figure 109-1 Supraventricular arrhythmias. A, Narrow QRS supraventricular tachycardia in a 4-month-old infant. Note the P wave after narrow QRS complex. B, Wide QRS supraventricular tachycardia secondary to aberrant conduction in an infant. There is no P wave visible. C, Permanent junctional reciprocating tachycardia. Note the negative P wave in leads II, III, and aVF, and the long R-P interval. D, Ectopic atrial tachycardia. Note the narrow complex tachycardia with P before the QRS complex with long PR interval and nonsinus axis. E, Atrial flutter in a neonate at an atrial rate of 428 beats/min with 2:1 AV conduction. F, Intraatrial reentrant tachycardia with 3 : 1 AV conduction and distinct atrial complexes in a post-Fontan patient. G, Junctional ectopic tachycardia in a 1-month-old infant with a more rapid narrow QRS junctional rate than atrial rate. A specific form of AVRT using a concealed accessory pathway, called permanent junctional reciprocating tachycardia (PJRT), is generally located in the posterior septal region. These pathways have negative P waves in leads II, III, and aVF, secondary to long refractory periods with slow retrograde conduction that results in a long RP interval. PJRT usually is an incessant arrhythmia, refractory to single-drug medical therapy. It may be confused with ectopic atrial tachycardia (especially those arising from the low right atrium) secondary to the ECG findings (see Figure 109-1, C). Wolff-Parkinson-White syndrome implies an accessory pathway between the atrium and the ventricle that usually has bidirectional conduction properties and associated episodes of SVT. The ECG reading is distinctive, with a short PR interval and a delta wave. The reported incidence is 1 to 4 per 1000 live births. Associated cardiac abnormalities include Ebstein anomaly of the tricuspid valve and l-transposition of the great arteries. In addition, WPW may be seen in association with cardiac rhabdomyoma and with hypertrophic cardiomyopathy. WPW most commonly presents in infancy with AVRT. Although many patients will have resolution of this arrhythmia after the first year of life, persistence of the WPW pattern after 5 years of life predicts continued episodes of SVT in 78% of patients.10 Antidromic tachycardia using the accessory pathway as the antegrade limb occurs in approximately 10% of children, with one half of these having multiple accessory pathways.10 The remainder use the AV node as the antegrade pathway and are termed orthodromic. If the WPW pattern persists and symptoms are present in older children, risk stratification should be performed including an exercise stress test, 24-hour ambulatory monitoring, and, possibly, an electrophysiological study. The presence of a short refractory period (<220 to 250 ms) or a shortest preexcited RR interval during atrial fibrillation (<220-250 ms) or multiple pathways increases the risk of a subsequent life-threatening event, and catheter ablation is advised.15 In addition, the presence of atrial fibrillation, present in 10% to 12% of children, can increase the risk of sudden cardiac death (SCD) because of rapid ventricular response.15 Although most children with WPW who have symptoms will have episodes of rapid heart rate or complain of palpitations, a small percentage will present with sudden cardiac death as the first manifestation.16 Asymptomatic patients may be noted to have the WPW pattern on an ECG obtained for other reasons. Concerning studies by Pappone and Santinelli show an increased risk for sudden death in WPW syndrome in association with short accessory pathway–effective refractory periods and with multiple pathways, and they recommend that catheter ablation be performed.15,17 A recent Pediatric and Congenital Electrophysiology Society/Heart Rhythm Society consensus statement on the management of asymptomatic WPW ECG patterns has been published.18 The recommendations for ablation are largely based on finding the shortest preexcited RR interval during atrial fibrillation (<220 to 250 ms). Treatment for patients with WPW syndrome varies based on the patient’s age and symptoms. Antiarrhythmic medications are used predominantly in infants and young children with WPW. Infrequently, an infant who presents with a narrow complex tachycardia will develop evidence of preexcitation after treatment with a medication such as digoxin that slows AV nodal conduction. β-Blockers are generally used as a first-line therapy unless there is a contraindication, such as severe reactive airway disease. When β-blocker therapy fails to control the tachycardia, other medications such as flecainide, amiodarone, or sotalol can be used. Digoxin or calcium-channel blockers should not be used in patients with WPW because of the potential for enhancement of conduction down the accessory pathway, along with block of the conduction through the AV node.19 This can lead to an increased risk of rapid conduction of atrial fibrillation or PACs through the accessory pathway. If a neonate with WPW syndrome presents with significant signs of cardiac decompensation with cardiogenic shock, digoxin can be used while the patient is still in the hospital to improve ventricular function, as well as to suppress the tachycardia. Transition to another antiarrhythmic medication other than digoxin should occur before discharge from the hospital. The AV nodal region comprises the reentry circuit in AVNRT. It occurs more commonly in young adults and adolescents than in younger children and is infrequently seen in patients during the neonatal period because dual AV nodal pathways become increasingly manifest as the child ages.21 The ECG usually reveals tachycardia in which there is a very short R-P interval on the ECG or no visible P wave representing typical AVNRT because the retrograde limb of the pathway conducts very rapidly from the ventricle to the atrium. Atypical AVNRT with a long R-P indicating a slow-slow circuit is less commonly seen. Treatment is aimed at the AV node with the most commonly used drugs being digoxin or β-blockers, with calcium-channel blockers only used in older children and generally if the other drugs are ineffective. Slow pathway modification is a reasonable approach in the school-age child, but the potential for AV block and the subsequent need for a pacemaker require skill and availability of all modern techniques and technology, as well as recognition of the position of AV nodal structures in the smaller heart. Newer cryoablation techniques may decrease the incidence of AV block but have a somewhat increased recurrence rate of 6.5% within 1 to 2 years after ablation.22 Tachycardia originating from the nonsinus portion of either atrium is designated ectopic atrial tachycardia (EAT) and represents approximately 10% of the SVT seen in the overall population. The heart rates in ectopic atrial tachycardias will be inappropriately fast for the patient’s activity level and are in the 130 to 250 beats/min range. The counted pulse rate may not be the same as the atrial rate because of blocked atrial conduction through the AV node. Automatic foci can be found in all areas of the atria. The sites of the atrial tachycardias include the atrial appendage, tricuspid annulus, and crista terminalis on the right side, and the pulmonary veins, mitral annulus, and posterior left atrium on the left side.23 The mechanism of ectopic atrial tachycardia is most often automatic but may be triggered or microreentry, recently labeled nonautomatic focal atrial tachycardia. Automatic atrial tachycardias are secondary to enhanced automaticity and occur spontaneously, or they can be induced by isoproterenol infusion and are often suppressed by sedation. Ectopic atrial tachycardia is not frequently seen in young infants, representing only 18% of all SVT seen in children in one report.24 With EAT, there is a gradual increase in heart rate and slowing on termination, which is markedly different from the sudden onset and offset seen in reentrant SVT, making it more difficult for the patient to recognize the tachycardia. The incessant nature of atrial tachycardias and the difficulty in perception of the arrhythmia can lead to significant depression of myocardial function, which results in a tachycardia-mediated cardiomyopathy.25 Cardiomyopathy resolution can take 6 to 12 months after the tachycardia has been effectively treated. The ECG reading generally shows a narrow complex SVT with a P wave of nonsinus origin preceding the QRS complex (see Figure 109-1, D). Nonautomatic focal atrial tachycardia (NAFAT) originates in a delimited area with activation of the remainder of the atrium emanating from this region. There is some overlap of terminology in the literature, with some defining NAFAT as all ectopic foci but, more specifically, that NAFAT is different from automatic atrial tachycardia in that it can be induced and terminated by pacing and appears to have a triggered or microreentrant mechanism. Although NAFAT in adults occurs most commonly in structurally normal hearts, in children or young adults, it is most often seen in association with congenital heart disease. The majority of NAFAT sites are in the right atrium and amenable to catheter ablation, with a relatively high success rate.26 They are difficult to differentiate from intraatrial reentrant tachycardia or atrial flutter on surface ECG. Atrial flutter (AF) is a reentry circuit in the atria, with the majority of pediatric atrial flutter seen in patients with congenital heart disease. The typical ECG findings in “classic” atrial flutter include negative P waves in the inferior leads (II, III, and aVF), with atrial rates of 250 to 450 bpm (see Figure 109-1, E). Atrial flutter represents 15% to 50% of all fetal SVT, often resulting in fetal hydrops, a form of congestive heart failure. Sotalol provides effective treatment in 80% of fetuses with atrial flutter.27 Neonates with atrial flutter usually have a structurally normal heart, although congenital heart disease may be present. Heart failure was present in 20% of 50 infant cases with an atrial rate range of 340 to 580 bpm.28 Atrial or supraventricular arrhythmias may be encountered in follow-up. In the neonate, treatment to convert AF to a sinus rhythm includes medication, transesophageal pacing, or direct current cardioversion. In patients with congenital heart disease, AF may develop before or after surgical interventions and is most commonly seen in lesions with extensive atrial surgery or atrial dilatation. The multiple atrial flutter circuits seen after congenital heart surgery result in slower rates and unusual flutter wave axis and morphology, often described as intraatrial reentry tachycardia rather than atrial flutter (see Figure 109-1, F). Symptoms are related to the ventricular rate during the tachycardia and the underlying health of the myocardium. To treat AF and prevent emboli, the presence of atrial thrombi should be ruled out before attempting conversion of the rhythm. An associated congenital heart defect such as an undiagnosed atrial septal defect can be evaluated at the same time. Catheter ablation is an effective treatment in patients with recurrent AF who are beyond early childhood, with success in 91% of patients.29 Atrial fibrillation is a primary atrial tachycardia that involves a number of microreentry circuits in the atrium. Adolescents with structurally normal hearts may present with idiopathic atrial fibrillation, and many of them will not have recurrences once the rhythm is converted; those who do have recurrences will require anticoagulation and antiarrhythmics. In young patients with AF and atrial fibrillation, the ECG after conversion should be carefully evaulated to rule out ST-segment changes associated with Brugada syndrome or short QT intervals because of the association of these arrhythmias. Similarly, in individuals with WPW and inducible atrial fibrillation, spontaneous clinical atrial fibrililation occurred in 73% and subsequent ventricular fibrillation occurred in 27%.30,31 Multifocal atrial tachycardia (MAT), or chaotic atrial tachycardia (CAT), is a primary atrial tachycardia with at least 3 distinct P-wave morphologies arising from multiple foci in the atria. It is occasionally confused with atrial fibrillation in that there are multiple P-wave morphologies and variable PR and R-R intervals. It is most commonly seen in the newborn, with spontaneous resolution over time in the majority.32 The management strategies are similar to those used in the treatment of EAT, but catheter ablation has not been useful because of the multiple foci. Yeager et al.33 reported a 17% incidence of sudden death in patients with MAT, possibly associated with bradycardia related to aggressive therapy. Two distinct types of junctional ectopic tachycardia (JET), an automatic tachycardia that arises from the AV junction, are seen in childhood. One is a familial form occurring early in infancy. The second occurs in the early postoperative period after congenital heart repair. In familial JET, heart rates range from 180 to 240 bpm, with a faster ventricular rate than the atrial rate and a narrow QRS complex (see Figure 109-1, G). Rarely, a rate-related aberrancy is seen, leading to a wide-complex tachycardia. If the QRS complex is wide, the diagnosis of VT must be considered. JET is characterized by a narrow QRS tachycardia at a rate of 170 to 300 bpm, with the atrial rate slower than the ventricular rate. Sudden death has been reported in this patient population.34,35 Treatment for the familial form of JET includes digoxin to slow the rhythm and improve ventricular function or amiodarone to slow the rate and/or convert the rhythm. Patients with JET have been treated with cathether ablation, with a 80% to 85% success rate, but with a risk of AV block, leading to recommendations for the use of cryothermal energy.36 In some patients, more commonly males, the junctional rate will slow over time and the patient may be weaned from taking long-term medications. Ventricular arrhythmias are less common than supraventricular arrhythmias in children. Because postoperative survival after complex surgery has increased, an increase in VT has been noted in these patients.37 The most common etiologies of VT in children include congenital heart disease, preoperatively and postoperatively; acquired heart disease such as myocarditis, cardiomyopathies including hypertrophic and dilated cardiomyopathies, ARVC, and left ventricular noncompaction (LVNC); coronary anomalies or disease resulting in myocardial ischemia; channelopathies such as congenital LQTS and catecholaminergic polymorphic ventricular tachycardia (CPVT); abnormal foci or circuits in structurally normal hearts; tumors or infiltrates; or idiopathic etiologies. Although the failure to identify a specific cause is not unusual in children, persistence in evaluations often identifies pathology in patients initially thought to have an unidentifiable etiology. Polymorphic VT includes torsades de pointes and bidirectional VT. Torsades de pointes is associated with LQTS. Bidirectional VT has been associated with familial hyperkalemic paralysis, CPVT, or digoxin toxicity. The mechanisms of VT in children include abnormal automaticity in 40% and reentry in 60%.38 Reentry is most often the mechanism in postoperative congenital heart patients, with reentry circuits that develop around suture lines, ventriculotomy scars, and anatomic obstacles. Symptoms appear to be rate related, most frequently seen with VT rates over 150 bpm. Patients with VT and heart disease often have symptoms, whereas only one-third of patients with normal hearts and VT have symptoms. Sudden death occurs most commonly in the presence of an abnormal heart but has been reported in patients with normal hearts.39 Evaluation of VT should include ECG, 24-hour ambulatory monitoring, echocardiography, and exercise stress testing in patients older than 5 years of age who can cooperate during the procedures.
Arrhythmias in Pediatric Population
Pediatric Arrhythmias
Interpreting Pediatric Rhythms
Benign Common Pediatric Arrhythmias
Supraventricular Tachycardia
Arrhythmia
Agent
Dose (Oral)
SVT
Digoxin
Dose is age dependent
Give in 3 doses (1/2 TDD, 1/4 TDD, 1/4 TDD)
Preterm infant: 10-20 µg/kg TDD
Term newborn to adolescent: 30-40 µg/kg TDD
Oral to maximal TDD of 1-1.5 mg IV dose is 3/4 oral dose)
Oral maintenance: 10 µg/kg/day divided q12h
Verapamil
2-8 mg/kg/day ÷ tid
VT
Mexiletine
5-15 mg/kg/day divided q8h
SVT or VT
Propranolol
0.5-2 mg/kg/dose q6h
Nadolol
0.25 mg/kg/dose q12h
Atenolol
0.5-1 mg/kg/day qd
Flecainide
50-200 mg/m2/day or 3-6 mg/kg/day divided q12h
Amiodarone
Loading dose: 10-20 mg/kg/day divided q12h × 7 days
Maintenance: 5-10 mg/kg/dose qd
Sotalol
2-8 mg/kg/day divided q12h
Atrioventricular Reentrant Supraventricular Arrhythmias
Permanent Junctional Reciprocating Tachycardia
Wolff-Parkinson-White Syndrome
Atrioventricular Nodal Reentrant Tachycardia
Atrial Tachycardia
Ectopic Atrial Tachycardia
Focal Atrial Tachycardia
Atrial Flutter
Atrial Fibrillation
Multifocal Atrial Tachycardia or Chaotic Atrial Tachycardia
Junctional Ectopic Tachycardia
Postoperative Junctional Ectopic Tachycardia
Ventricular Arrhythmias
Ventricular Tachycardia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Arrhythmias in Pediatric Population
