complexities posed by limitations of venous access or surgical baffles or conduits. Patients with double-outlet right ventricle with subaortic conus can harbor an accessory connection in the subaortic conus between the discontinuous aortic annulus and mitral annulus, a situation that does not exist in normal hearts where the ablative techniques have been standardized. Patients with heterotaxy syndrome with either a functionally univentricular heart or two separate ventricles may have absence of the coronary sinus, presence of a left superior vena cava, separate atrial entry of the hepatic veins, and/or juxtaposition of the atrial appendages, in addition to surgical baffles limiting access to regions of the atria. Patients with congenitally corrected transposition of the great arteries (ccTGA) have a propensity to display multiple accessory connections in association with Ebstein’s anomaly of the systemic tricuspid valve. In addition to these anatomic variants, repaired tetralogy patients have a high incidence of ART caused by atrial dilatation and scar formation, as well as VT in the area of the ventricular septal defect closure and right ventriculotomy. Transcatheter ablation in the older Fontan patient is hampered by both the multiplicity of arrhythmia mechanisms and circuits, location of some arrhythmia circuits in the left atrium or under surgical patches over the atrial isthmus, as well as the marked atrial hypertrophy limiting the ability to create transmural conduction block. The contemporary congenital heart surgeon should have a comprehensive understanding of all arrhythmia types and the potential methods of ablation, as well as hurdles of anatomic complexity for arrhythmia surgery if a cardiac operation or reoperation for an anatomic repair/re-repair is required in a patient with arrhythmias.
Table 105.1 Arrhythmia Surgery and Congenital Heart Disease Repair: Children’s Memorial Hospital and the Cleveland Clinic Children’s Hospital Experience 1987 to 2010 (n = 248) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Table 105.2 Arrhythmia Types | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Table 105.3 Associated Diagnoses | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
circuits in the atria, while preserving intact AV conduction and atrial transport function, and may be performed using a combination of resection and linear ablative lesions. The right atrial maze procedure is performed for atrial reentry or atrial flutter, and both a right and left atrial maze procedure is performed for AF. The right atrial maze procedure alone is not adequate to eliminate AF, which is primarily of left atrial origin.
Table 105.4 Types of Arrhythmias | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
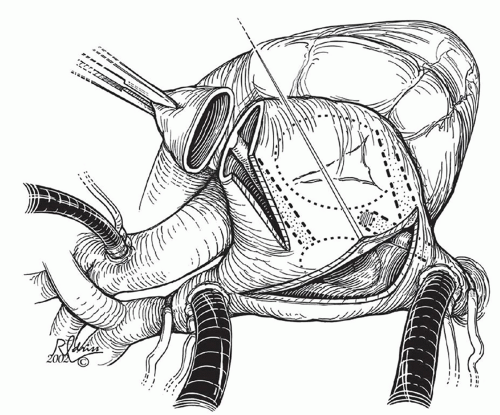
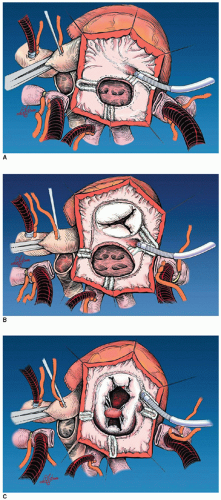
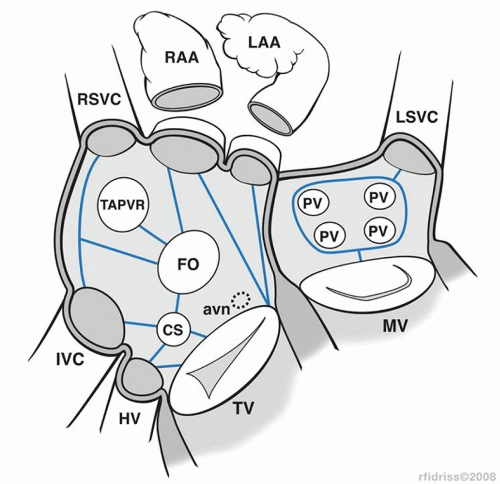
AV valve, coronary sinus, atrial appendage, and the fossa ovalis or atrial septal patch. When anatomic barriers are absent or anomalous, creative application become necessary by delivering ablative lesions using the guidelines mentioned previously. For example, since there is no tricuspid valve in tricuspid atresia, cryoablation lesions are applied as noted in Figure 105.2A. Figure 105.2B and 105.2C demonstrates the lesions that are required for single right ventricle/mitral atresia and functionally univentricular hearts with unbalanced AV canal, respectively. Figure 105.3 is a complex anatomic diagrammatic representation of the potential anomalies of systemic and pulmonary venous return, juxtaposed atrial appendages and proposed lines of block that are recommended for right- and left-sided maze procedures. The ablative lesions noted in Figure 105.3 are designed to be guidelines that are executed based on the preoperative electrophysiologic study and the type of arrhythmia that has been characterized. As an example, biatrial maze operations are performed if suture lines extend into the left atrium or if there is anomalous left superior vena caval drainage.
areas of focal tachycardia may require electrical anatomic isolation. Favorable outcomes have been achieved with cryoablation and excision of automatic foci. Automatic right atrial foci are often localized to the right atrial appendage and can be resected. Multiple ectopic foci resulting in arrhythmia recurrence stimulated surgeons to use more extensive and creative techniques such as pulmonary vein isolation, left atrial isolation, right atrial isolation, and His bundle ablation with pacemaker insertion in difficult cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree